碳化钛(TiC)基纤维二氧化硅催化剂的工程电子带结构平衡同时去除六价铬和四环素
IF 8.4
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
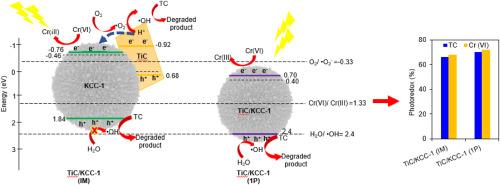
同时脱除废水中的四环素(TC)和六价铬(Cr(VI))受到光催化效率低和复杂催化剂回收的限制。本研究研究了碳化钛(TiC)基纤维二氧化硅KAUST催化中心(KCC-1)复合材料在可见光驱动下去除TC和Cr(VI),重点研究了煅烧、TiC负载(1-5 wt%)和合成方法的影响。未煅烧的TiC/KCC-1比煅烧的催化剂具有更高的表面积、锐钛矿含量和更强的TiC - support相互作用。与浸渍催化剂(TiC/KCC-1 (IM))相比,3TiC/KCC-1的去除率最高(68% Cr(VI), 66% TC),而一炉合成的TiC/KCC-1 (1P)表现出更好的性能(73% Cr(VI), 72% TC),更低的能耗(731 kWh/m3)和成本(36.7美元)。活性的增强归因于其窄带隙(1.7 eV)、有效的电荷分离和有利的能带位置。电化学研究表明,TiC/KCC-1 (1P)改善了电荷转移,降低了电阻。机制上,TiC/KCC-1 (1P)通过其+0.70 eV的导带引导光电子还原Cr(VI),而其+2.4 eV的价带通过羟基自由基形成支持TC氧化。相比之下,TiC/KCC-1 (IM)由于其不利的能带位置而受到电子竞争和氧化电位不足的影响。这些发现强调了TiC/KCC-1 (1P)是一种有前途的、绿色的、节能的多污染物废水处理光催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Engineered electronic band structure of titanium carbide (TiC)-based fibrous silica catalyst for balanced simultaneous removal of hexavalent chromium and tetracycline
Simultaneous removal of tetracycline (TC) and hexavalent chromium (Cr(VI)) from wastewater is limited by low photocatalytic efficiency and complex catalyst recovery. This study investigates titanium carbide (TiC)-based fibrous silica KAUST Catalysis Centre (KCC-1) composites for the visible-light-driven removal of TC and Cr(VI), focusing on the effects of calcination, TiC loading (1–5 wt%), and synthesis method. Uncalcined TiC/KCC-1 outperformed the calcined catalyst due to its higher surface area, anatase content, and stronger TiC–support interaction. Among loadings, 3TiC/KCC-1 achieved the highest removal (68 % Cr(VI), 66 % TC), while one-pot synthesized TiC/KCC-1 (1P) showed superior performance (73 % Cr(VI), 72 % TC), lower energy demand (731 kWh/m3), and cost (USD 36.7) compared to the impregnated catalyst (TiC/KCC-1 (IM)). Enhanced activity is attributed to its narrow band gap (1.7 eV), efficient charge separation, and favorable band positions. Electrochemical studies revealed TiC/KCC-1 (1P) improved charge transfer and reduced resistance. Mechanistically, TiC/KCC-1 (1P) directs photogenerated electrons toward Cr(VI) reduction via its +0.70 eV conduction band, while its +2.4 eV valence band supports TC oxidation through hydroxyl radical formation. In contrast, TiC/KCC-1 (IM) suffers from electron competition and insufficient oxidative potential due to its less favorable band positions. These findings underscore TiC/KCC-1 (1P) as a promising, green, energy-efficient photocatalyst for multi-contaminant wastewater treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Environmental Management
环境科学-环境科学
CiteScore
13.70
自引率
5.70%
发文量
2477
审稿时长
84 days
期刊介绍:
The Journal of Environmental Management is a journal for the publication of peer reviewed, original research for all aspects of management and the managed use of the environment, both natural and man-made.Critical review articles are also welcome; submission of these is strongly encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


