协同调谐离子液体负载氧化还原活性氧化钴/聚苯胺电催化剂促进分子析氢
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
开发具有成本效益、在宽pH范围内具有高活性的双功能电催化剂对于规模化制氢至关重要。钴基催化剂虽然很有前途,但往往受到导电性差、界面电阻和活性位点不稳定的限制。在这里,我们报道了一种原位工程杂化电催化剂,由还原氧化石墨烯(rGO)、氧化钴(Co3O4)和聚苯胺(PANI)组成,与离子液体1-乙基-3-甲基咪唑双(三氟甲基磺酰基)亚胺(EMIM TFSI)协同掺杂。这种离子掺杂调节了界面质子转移,增强了离子传输,改善了表面润湿性,增强了电化学稳定性和活性。混合材料(r-Co3O4/P)在0.1 M H2SO4和0.1 M KOH中,在10 mA cm−2下分别表现出158 mV和176 mV的低过电位,具有超过10,000次循环的优异耐久性。在100 mV过电位下,转换频率为1.56 s−1(酸性)和0.32 s−1(碱性),具有较强的本征析氢活性。这些结果确立了离子液体掺杂杂化催化剂作为下一代水电解系统中可行的双功能HER候选物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
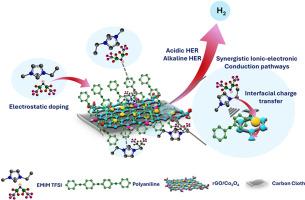
Synergistically tuned ionic liquid supported redox active cobalt oxide/PANI electrocatalyst for boosting molecular hydrogen evolution
Developing cost-effective, bifunctional electrocatalysts with high activity across a broad pH range is critical for scalable hydrogen production. Cobalt-based catalysts, while promising, are often limited by poor conductivity, interfacial resistance, and instability of active sites. Here, we report an in-situ engineered hybrid electrocatalyst comprising reduced graphene oxide (rGO), cobalt oxide (Co3O4), and polyaniline (PANI), synergistically doped with the ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMIM TFSI). This ionic doping modulates interfacial proton transfer, enhances ion transport, and improves surface wettability, enhancing electrochemical stability and activity. The hybrid (r-Co3O4/P) exhibits low overpotentials of 158 mV and 176 mV at 10 mA cm−2 in 0.1 M H2SO4 and 0.1 M KOH, respectively, with excellent durability over 10,000 cycles. It achieves turnover frequencies of 1.56 s−1 (acidic) and 0.32 s−1 (alkaline) at 100 mV overpotential, demonstrating strong intrinsic hydrogen evolution activity. These results establish ionic liquid-doped hybrid catalysts as viable bifunctional HER candidates for next-generation water electrolysis systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


