通过靶向LBP突变,揭示携带N-(4-(苯氧基)苯基)哌啶-1-磺胺支架的AR拮抗剂在前列腺癌治疗中的疗效
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
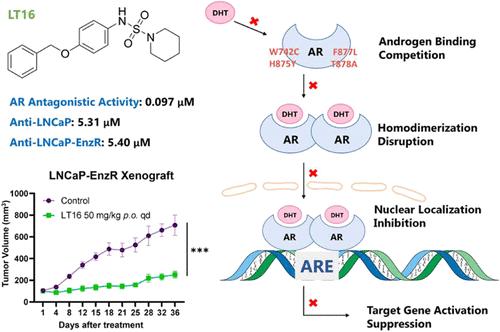
雄激素受体(AR)的点突变是前列腺癌(PCa)耐药的重要驱动因素,对开发有效的治疗策略提出了巨大的挑战。在我们之前发现的次优AR拮抗剂T1-12的基础上,我们通过结构优化和对t878a突变AR的综合筛选,开发了LT16,它含有N-(4-(苯氧基)苯基)哌啶-1-磺胺支架。LT16通过充分拮抗临床AR突变并有效抑制去势和enzalutamide耐药LNCaP细胞的体外增殖,优于现有的抗雄激素。机械上,LT16被发现破坏AR核易位,阻碍AR同二聚体化,并通过与配体结合袋的竞争性结合抑制AR调节基因的转录。进一步的体内实验表明,LT16显著降低小鼠常规和enzalutamide耐药LNCaP肿瘤体积和血清前列腺特异性抗原水平。这些发现使LT16成为晚期PCa的一种有前景的创新治疗方法,特别是在对当前治疗方法产生耐药性的情况下。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unraveling the Efficacy of AR Antagonists Bearing N-(4-(Benzyloxy)phenyl)piperidine-1-sulfonamide Scaffold in Prostate Cancer Therapy by Targeting LBP Mutations
Point mutations in the androgen receptor (AR) are significant drivers of resistance in prostate cancer (PCa), posing a great challenge to the development of effective treatment strategies. Building on our previous discovery of the suboptimal AR antagonist T1-12, we developed LT16, which contains an N-(4-(benzyloxy)phenyl)piperidine-1-sulfonamide scaffold through structural optimization and comprehensive screening against T878A-mutated AR. LT16 outperformed existing antiandrogens by fully antagonizing clinical AR mutations and effectively suppressing castration- and enzalutamide-resistant LNCaP cells proliferation in vitro. Mechanically, LT16 was found to disrupt AR nuclear translocation, hinder AR homodimerization, and suppress transcription of AR-regulated genes by competitive binding to the ligand binding pocket. Further in vivo experiments demonstrated that LT16 significantly reduced both regular- and enzalutamide-resistant LNCaP tumor volume and serum prostate-specific antigen levels in mice. These findings position LT16 as a promising and innovative therapeutic for advanced PCa, particularly in cases where resistance to current therapies is a concern.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


