Pb(II)在赤铁矿(001),(116),和(104)表面的面依赖性吸附
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
赤铁矿常见的(001)和(012)面经常用于铅(Pb)吸附行为的模型研究,但缺乏对自然界中存在的高能面(104)的研究。此外,很少有研究试图将特定方面的铅吸附的分子细节与宏观摄取行为联系起来。为了解决这些知识空白,我们研究了(001)、(104)和(116)主导的表面工程赤铁矿纳米颗粒对Pb(II)的吸附行为。吸附实验表明,三种样品对Pb(II)的吸收量存在显著差异,其中(001)的吸收量最高,(116)的吸附效率最高。吸附动力学服从拟二阶模型,表明吸附过程主要受化学吸附控制。Langmuir模型很好地拟合了吸附等温线,表明吸附继续进行,直到大约单层吸附。详细的表征表明,Pb(II)作为单个原子被吸附,具有复杂的内球结合模式,在不同的方面变化,表明吸附既依赖于结构,也依赖于能量方面。共吸附实验进一步证明,Cu2+、Zn2+和腐植酸对Pb(II)的吸附有显著促进作用。该研究促进了对赤铁矿表面反应性控制宏观湿吸附行为的认识,为Pb(II)的环境命运提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
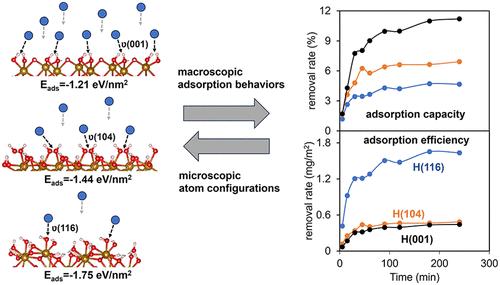
Facet-Dependent Adsorption of Pb(II) on Hematite (001), (116), and (104) Surfaces
Hematite’s common (001) and (012) facets are frequently used in model studies of lead (Pb) adsorption behavior, but there is a lack of research on the high-energy facets, e.g., (104), present in nature. Also, few studies have attempted to connect the molecular details of facet-specific Pb adsorption to the macroscopic uptake behavior. To address these knowledge gaps, we investigated Pb(II) adsorption behaviors on facet-engineered hematite nanoparticles dominated by (001), (104), and (116). Adsorption experiments revealed significant variations in Pb(II) uptake among the three samples, with (001) demonstrating the highest capacity and (116) showing the best adsorption efficiency when normalized to the specific surface area. Adsorption kinetics followed the pseudo-second-order model, indicating that the adsorption process is governed mostly by chemisorption. Adsorption isotherms were well fitted by the Langmuir model, indicating that uptake proceeds until roughly monolayer adsorption. Detailed characterization revealed Pb(II) was adsorbed as single atoms with complex inner-sphere binding modes that varied across different facets, indicating that adsorption is both structurally and energetically facet-dependent. Coadsorption experiments further demonstrated Cu2+, Zn2+, and humic acid significantly promoted Pb(II) adsorption. This study advances the understanding of hematite surface reactivity in controlling macroscopic wet adsorption behaviors, providing valuable insights into the environmental fate of Pb(II).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


