基于oa的光fenton系统产生氧化自由基和还原自由基,同时去除地表水中的1-NA和Cr (VI)
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
还原自由基的产生不足限制了光- fenton系统去除有机污染物和重金属的能力,同时活化剂的成本限制了它们的实际应用。本文以草酸(OA)为活化剂,与MIL-88A(Fe) (M88A(Fe)) (M88A(Fe))组成了低成本的光fenton (M88A(Fe)/OA/Vis)体系。在该体系中,OA的加入使1-萘胺(1- na) (Kobs=0.7442 min−1)和Cr (VI) (Kobs=2.6734 min−1)的去除率分别提高了226.88倍和106.64倍。此外,在较宽的pH范围(3-11)和复杂干扰物质(Cl−、NO3−、SO42−、HCO32−、H2PO4−、腐植酸、自来水、湖水和河水)的存在下,1-NA和Cr (VI)的去除率达到90%以上。原位ATR-FTIR光谱和理论分析表明,Fe(C2O4)+由OA和Fe位点之间的配体交换形成,并通过非还原性溶剂快速解离转化为Fe(C2O4)2−。在可见光下,Fe(C2O4)2−被一个空穴还原生成Fe(C2O4)+和·C2O4−,·C2O4−通过C-C键断裂迅速生成·CO2−。同时,水和氧分别与电子和空穴反应生成·OH和·O2−,从而实现两种污染物的高效氧化还原。此外,所开发的催化膜反应器连续运行12 h, 1-NA去除率达到95%以上,Cr (VI)去除率达到100%。我们的研究结果证明了OA在水净化中的优势,为基于有机酸的生态友好型水净化技术的发展铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。

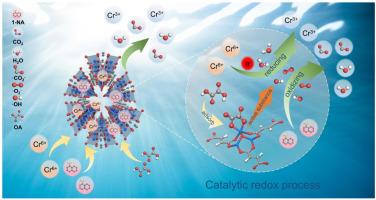
OA-based photo-Fenton system generates oxidizing and reducing radicals for the simultaneous removal of 1-NA and Cr (VI) in surface waters
The inadequate generation of reducing radicals limits the ability of photo-Fenton systems to remove organic pollutants and heavy metals at the same time, meanwhile the cost of the activators limits their practical application. Here, we used oxalic acid (OA) as an activator with MIL-88A(Fe) (M88A(Fe)) to form a low-cost photo-Fenton (M88A(Fe)/OA/Vis) system. In this system, OA accession resulted in a remarkable 226.88 and 106.64-fold enhancement in the removal of 1-naphthylamine (1-NA) (Kobs = 0.7442 min−1) and Cr (VI) (Kobs = 2.6734 min−1), respectively. Furthermore, over 90 % removal of 1-NA and Cr (VI) were achieved in a wide pH range (3-11) and the presence of complex interfering substances (Cl−, NO3−, SO42−, HCO32−, H2PO4−, humic acid, tap water, lake water, and river water). In situ ATR-FTIR spectra and theoretical analysis revealed that Fe(C2O4)+ is formed by ligand exchange between OA and Fe sites and is rapidly dissociated via nonreducing solvents and converted to Fe(C2O4)2−. Under visible light, Fe(C2O4)2− is reduced by a hole to give Fe(C2O4)+ and ·C2O4−, and the ·C2O4− quickly forms ·CO2− through C-C bond breaking. Meanwhile, ·OH and ·O2− are produced by the reaction of water and oxygen with electrons and holes, respectively, thus realizing the efficient redox of two pollutants. Moreover, the catalytic membrane reactor developed was operated continuously for 12 h to remove more than 95 % of 1-NA and 100 % of Cr (VI), respectively. Our findings demonstrate the advantages of OA in water decontamination and pave the way for the development of eco-friendly water decontaminations based on organic acids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


