探索盐酸浸出中等品位磷矿中磷解离动力学:迈向高质量磷酸二钙生产
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
反应动力学对于理解过程机制至关重要,在磷提取的回收中起着关键作用,为优化和扩大规模提供了重要的见解,这直接影响到盈利能力和工程设计。本文重点对制备的盐酸从磷灰石矿物中提磷的反应动力学进行了综合研究,强调了其对资源高效利用和工艺改进的意义。对酸浓度、固液比、粒径分数、搅拌速率和温度对溶解速率的影响进行了细致的评估。采用非均相反应模型研究了磷灰石萃取磷的溶解动力学,确定了最适合实验数据的方程。在14℃条件下,P2O5的提取率为99.93%,颗粒为(+0.063 ~ 0.15)mm,萃取时间为90 s。计算出典型样本的方差系数(CV)为112%。采用收缩核模型(SCMs)确定了浸出过程的速率控制阶段,得到了P2O5浸出的半经验动力学方程。用阿伦尼乌斯方程计算得到的活化能为15.88 kJ/mol。能量色散x射线光谱(EDS), x射线衍射(XRD)和傅里叶变换红外光谱(FT-IR)分析证实了二氧化硅残留物。这项工作阐明了磷提取动力学,旨在优化工艺条件,设计高效的反应器,并评估操作的经济可行性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
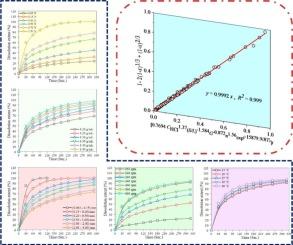
Exploring phosphorus dissociation kinetics in HCl-leached moderate-grade phosphate ores: Towards high-quality dicalcium phosphate production
Reaction kinetics is crucial for understanding process mechanisms and plays a key role in the recovery of phosphorus extraction, providing essential insights for optimization and scaling up, which directly impact profitability and engineering design. This article focuses on the comprehensive study of the reaction kinetics involved in phosphorus extraction from apatite mineral using prepared HCl, emphasizing its significance for efficient resource utilization and process enhancement. The impacts of acid concentration, solid-to-liquid ratio, particle size fractions, agitation rate, and temperature on the dissolution rate were meticulously evaluated. The dissolution kinetics of phosphorus extraction from apatite using heterogeneous reaction models, identifying the best-fitting equation for experimental data. A P2O5 extraction rate of 99.93 % was achieved at 14 °C with (+0.063–0.15) mm particles after 90 s. The variance coefficient (CV) of the typical sample was calculated to be 112 %. Shrinking Core Models (SCMs) were employed to determine the rate-controlling phase of the leaching process, leading to a semi-empirical kinetic equation for P2O5 leaching. The activation energy was found to be 15.88 kJ/mol using the Arrhenius equation. Energy dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR) analyses confirmed silica residue. This work sheds light on phosphorus extraction kinetics, aiming at optimizing the process conditions, designing efficient reactors, and assessing the operation’s economic viability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


