SlLHP1b的钙响应磷酸化表观遗传抑制生长素合成以控制干旱诱导的番茄落花
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
干旱导致花和果实脱落,从而降低产量。尽管在赖氨酸27 (H3K27me3)依赖通路中组蛋白H3的三甲基化已被证明可以控制干旱反应,但干旱诱导脱落的机制尚不清楚。在这项研究中,我们发现干旱诱导的钙调神经磷酸酶b -样蛋白11 (SlCBL11)上调激活了与钙调神经磷酸酶相互作用的蛋白激酶10 (SlCIPK10),在番茄雄蕊中分别在Thr387和Thr389位点磷酸化样异染色质蛋白1b (SlLHP1b)。Thr389位点的磷酸化提高了SlLHP1b的稳定性,Thr387/Thr389位点的磷酸化增强了SlLHP1b与H3K27me3的结合能力。因此,sllhp1b依赖的SlYUC在雄蕊中表达的表观遗传沉默被促进,雄蕊中生长素含量降低,导致脱落区生长素梯度被破坏,最终导致番茄落花。总的来说,我们发现了Ca2+信号通过SlCBL11-SlCIPK10-SlLHP1b模块控制H3K27me3抑制干旱诱导落花过程中生长素合成的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
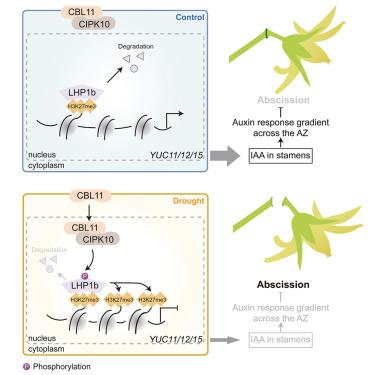
Calcium-responsive phosphorylation of SlLHP1b epigenetically suppresses auxin synthesis to control drought-induced flower drop in tomato
Drought causes the abscission of flowers and fruits, thereby reducing yields. Although trimethylation of histone H3 in the lysine 27 (H3K27me3)-dependent pathway has been shown to control drought responses, the mechanisms underlying drought-induced abscission remain unknown. In this study, we showed that drought-induced calcineurin B-like11 (SlCBL11) upregulation activated the CBL-interacting protein kinase10 (SlCIPK10) to phosphorylate like heterochromatin protein 1b (SlLHP1b) at Thr387 and Thr389, respectively, in stamens of tomato (Solanum lycopersicum). Phosphorylation at Thr389 improved SlLHP1b stability, and phosphorylation at Thr387/Thr389 enhanced SlLHP1b binding ability to H3K27me3. Hence, SlLHP1b-dependent epigenetic silencing of the expression of SlYUC in stamens was promoted, which decreased the auxin content in stamens, causing the disruption of the auxin gradient in the abscission zone, and eventually flower drop in tomatoes. Overall, we showed a mechanism by which Ca2+ signaling controls H3K27me3 through the SlCBL11-SlCIPK10-SlLHP1b module to inhibit auxin synthesis during drought-induced flower drop.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


