单细胞和空间转录组分析鉴定炎症性乳腺癌的细胞异质性和免疫抑制肿瘤微环境
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
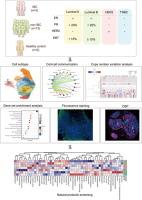
炎性乳腺癌(IBC)是一种高度侵袭性的乳腺癌亚型,预后较差。更好地了解IBC的病理和分子基础对于制定精准医疗策略至关重要。本研究旨在从单细胞和空间水平分析IBC,以检测免疫细胞群、信号通路,并确定治疗IBC的潜在治疗靶点。方法采用单细胞RNA测序(scRNA-seq)技术鉴定IBC和非IBC样本之间的免疫相关差异。利用qRT-PCR和荧光染色来验证scRNA-seq的结果,同时利用NanoString GeoMx数字空间分析器进行空间分析以评估免疫细胞浸润。通过肿瘤免疫细胞共培养试验来评估CXCL13的细胞毒性作用。通过体内研究来评估CXCL13对免疫治疗效果的影响。此外,对天然产物进行筛选,以确定治疗IBC的潜在免疫调节剂。结果scrna -seq显示IBC肿瘤微环境中T细胞中CXCL13表达显著降低,这一发现与较差的患者预后相关。此外,免疫相关基因组明显下调,细胞间相互作用减弱,表明IBC内存在免疫抑制状态。空间分析进一步表明,IBC肿瘤组织中cd45阳性免疫细胞的存在减少,突出了这种侵袭性癌症亚型的免疫浸润受损特征。最重要的是,CXCL13在肿瘤细胞中过表达,与免疫细胞共培养,显著促进肿瘤细胞死亡。CXCL13还能增强体内抗pd -1治疗的疗效。此外,对天然产物的筛选发现,血根碱和α-山竹苷是潜在的免疫调节化合物,为调节IBC的免疫反应和改善治疗结果提供了有希望的治疗途径。结论本研究结果揭示了IBC“冷”肿瘤微环境的内在异质性。这些因素共同促成了IBC的免疫抑制特征。此外,天然产物筛选发现血根碱和α-山竹苷是有前途的免疫调节剂,为改善治疗结果提供了潜在的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Single-cell and spatial transcriptome profiling identifies cellular heterogeneity and immunosuppressive tumor microenvironment in inflammatory breast cancer
Introduction
Inflammatory breast cancer (IBC) is a highly aggressive subtype of breast cancer associated with a poor prognosis. A better understanding of IBC’s pathological and molecular basis is crucial for developing precision medicine strategies.Objective
This study aimed to profile IBC at both the single-cell and spatial levels to examine immune cell populations, signaling pathways, and identify potential therapeutic targets for treating IBC.Methods
Single-cell RNA sequencing (scRNA-seq) was employed to identify immune-related differences between IBC and non-IBC samples. qRT-PCR and fluorescence staining were utilized to validate the findings from scRNA-seq, while spatial analysis using the NanoString GeoMx Digital Spatial Profiler was conducted to evaluate immune cell infiltration. Tumor-immune cell co-culture assays were conducted to assess the cytotoxic role of CXCL13. In vivo studies were performed to assess the effect of CXCL13 on the efficacy of immunotherapy. Furthermore, a screening of natural products was performed to identify potential immunomodulatory agents for the treatment of IBC.Results
scRNA-seq revealed a significant reduction in CXCL13 expression in T cells within the IBC tumor microenvironment, a finding that correlated with poorer patient outcomes. Additionally, immune-related gene sets were notably downregulated, and cell–cell interactions were diminished, indicating a state of immune suppression within IBC. Spatial analysis further demonstrated a reduced presence of CD45-positive immune cells within IBC tumor tissues, highlighting the compromised immune infiltration characteristic of this aggressive cancer subtype. Most importantly, overexpression of CXCL13 in tumor cells, under co-culture with immune cells, significantly promoted tumor cell death. CXCL13 can also enhance the efficacy of anti-PD-1 therapy in vivo. Furthermore, screening of natural products identified sanguinarine and α-mangostin as potential immunomodulatory compounds, offering promising therapeutic avenues for modulating the immune response in IBC and improving treatment outcomes.Conclusion
Our findings reveal inherent heterogeneity within the “cold” tumor microenvironment of IBC. These factors collectively contribute to the immune suppression characteristic of IBC. Additionally, natural product screening identified sanguinarine and α-mangostin as promising immunomodulatory agents, offering potential therapeutic strategies to improve treatment outcomes.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


