毒素转基因抑制dna传感增强NK细胞活性
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
自然杀伤(NK)细胞疗法已显示出强大的癌症治疗潜力;然而,NK细胞的功效受到其寿命短和肿瘤微环境抑制的限制。导致这种抑制的一个因素可能是环GMP-AMP合成酶(cGAS)途径的激活。自从发现cGAS作为DNA传感器以来,人们对DNA传感机制重新产生了兴趣,尽管DNA传感介导的天然免疫反应在NK细胞上的作用尚不清楚。毒素是一种来自DNA病毒的蛋白质,已知可以降解cGAS信号产物环GMP-AMP (cGAMP),并可能缓解NK细胞中DNA传感相关的应激。目的探讨Poxin表达是否能抑制NK细胞的DNA感应通路,从而增强NK细胞的细胞毒功能和抗肿瘤活性。方法制备表达Poxin基因的NK-92细胞,观察其对DNA感应激活的反应。通过外源质粒转染或辐照刺激cGAS-STING途径诱导内源性双链DNA (dsDNA)。我们通过细胞毒性实验、穿孔素和颗粒酶B表达水平分析来评估NK-92和脐带血源性原代NK (pNK)细胞的活性,通过RNA-seq分析来探索其作用机制,并通过功能实验来评估NK-92和嵌合抗原受体(CAR)-NK-92细胞的抗肿瘤功效。结果spoxin的表达显著抑制了cGAS-STING通路,降低了对质粒转染和辐照诱导的dsDNA的激活。RNA-seq分析表明,在表达poxin的NK-92细胞中,细胞毒性介质(包括穿孔素和颗粒酶B)水平升高。此外,与对照组相比,这些转基因NK-92/CAR-NK-92细胞表现出更强的抗肿瘤活性。结论毒毒素可有效抑制NK细胞DNA感知介导的先天免疫,增强NK细胞的细胞毒性和抗肿瘤活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
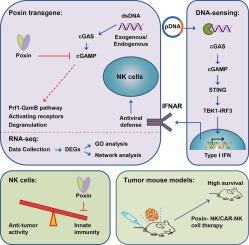
Enhancement of NK cell activity via DNA-sensing inhibition by Poxin transgene
Introduction
Natural Killer (NK) cell therapy has shown strong potential for cancer treatment; however, NK cell efficacy is limited by their short lifespan and suppression within the tumor microenvironment. One factor contributing to this suppression may be the activation of the cyclic GMP-AMP synthase (cGAS) pathway. Since the discovery of cGAS as a DNA sensor, there has been renewed interest in DNA sensing mechanisms, although the role of DNA sensing-mediated innate immune responses on NK cells remains unclear. Poxin, a protein derived from DNA viruses, is known to degrade the cGAS signaling product cyclic GMP-AMP (cGAMP) and could potentially alleviate DNA sensing-related stress in NK cells.Objectives
This study aims to investigate whether Poxin expression can inhibit DNA sensing pathways in NK cells, thereby enhancing their cytotoxic function and anti-tumor activity.Methods
We generated NK-92 cells expressing a Poxin transgene and evaluated their response to DNA sensing activation. The cGAS-STING pathway was stimulated through either exogenous plasmid transfection or irradiation to induce endogenous double-stranded DNA (dsDNA). We assessed NK-92 and umbilical cord blood derived primary NK (pNK) cells activity through cytotoxicity assay and analysis of perforin and granzyme B expression levels, performed RNA-seq analysis to explore the mechanism and conducted functional assays to evaluate anti-tumor efficacy of NK-92 and chimeric antigen receptors (CAR)-NK-92 cells.Results
Poxin expression significantly inhibited the cGAS-STING pathway, reducing activation in response to both plasmid transfection and irradiation-induced dsDNA. RNA-seq analysis indicated increased levels of cytotoxic mediators, including perforin and granzyme B, in Poxin-expressing NK-92 cells. Furthermore, these transgenic NK-92/CAR-NK-92 cells exhibited enhanced anti-tumor activity compared to controls.Conclusion
Poxin effectively suppresses DNA sensing-mediated innate immunity in NK cells, enhancing their cytotoxicity and anti-tumor effectiveness.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


