利用RGDS肽调节细菌-宿主界面用于靶向抗菌治疗
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
病原细菌,特别是耐多药细菌引起的难治性感染对全球人类健康构成重大威胁。新兴的以宿主为导向的抗微生物策略有可能延长现有治疗的有效性或消除抗生素耐药性的发展,这是解决耐药细菌引起的感染的一个有希望的途径。在这里,我们提出了一种基于机械生物学的宿主定向抗菌策略,该策略利用抑制纤维连接蛋白(Fn)肽Arg-Gly-Asp-Ser (RGDS)来调节细菌-宿主界面粘附力,从而有效地对抗多重耐药细菌感染。RGDS肽可以竞争性地干扰细菌-宿主粘附界面的物理化学相互作用,从而抑制细菌在宿主表面的定植。通过单细胞力谱法(SCFS)定量分析了RGDS肽调控的细菌-宿主粘附力的变化,从而阐明了宿主靶向抗菌策略的机械生物学机制。此外,我们揭示了RGDS肽作为抗生素佐剂的抗菌潜力,实现了75% %的抗生素用量减少。rgds增强的低剂量抗生素的体内抗菌效果也在三种动物模型中得到验证。基于机械生物学的宿主定向抗菌策略有望广泛应用于解决全球耐药细菌的发病率和进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。

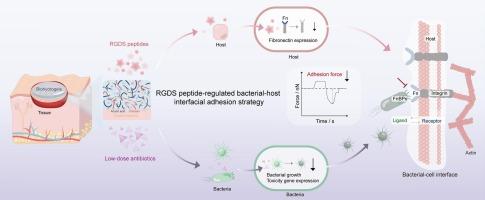
Harnessing RGDS peptides to regulate bacterial-host interfaces for targeted antimicrobial therapy
Refractory infections caused by pathogenic bacteria, particularly multidrug-resistant bacteria, pose a significant threat to global human health. Emerging host-directed antimicrobial strategies have the potential to prolong the effectiveness of existing treatments or eliminate the development of antibiotic resistance, representing a promising avenue for addressing infections caused by resistant bacteria. Here, we propose a mechanobiology-based host-directed antimicrobial strategy that utilizes an inhibitory fibronectin (Fn) peptide, Arg-Gly-Asp-Ser (RGDS), to regulate bacterial-host interfacial adhesion forces and thereby effectively combat multidrug-resistant bacterial infections. The RGDS peptides can competitively interfere with the physicochemical interactions at the bacterial-host adhesion interfaces, thereby inhibiting bacterial colonization on host surfaces. The alterations in bacterial-host adhesion forces regulated by RGDS peptides are quantified via single-cell force spectroscopy (SCFS), which elucidate the mechanobiological mechanisms underlying the presented host-targeted antimicrobial strategy. Furthermore, we reveal the antimicrobial potential of the RGDS peptide as an antibiotic adjuvant, achieving a 75 % reduction in antibiotic dosage. The in vivo antimicrobial efficacy of RGDS-enhanced low-dose antibiotics is also validated in three animal models. It is expected that a mechanobiology-based host-directed antimicrobial strategy could be broadly employed to address the global incidence and progression of drug-resistant bacteria.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


