长链脂肪酸对新生哺乳动物心脏心肌细胞成熟的代谢影响
IF 4.3
3区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
心肌细胞是心脏病建模、药物开发和再生治疗的重要模型。具体来说,人类诱导的多能干细胞衍生的心肌细胞(hiPSC-CMs)已经成为广泛使用的具有高重复性的细胞模型。然而,体外生成的心肌细胞在复制成人心肌细胞的电生理和机械功能方面往往仍然不成熟,不足,限制了这些模型的临床和实验应用。因此,人们探索了各种生物化学和生物物理策略来促进心肌细胞的成熟,以解决这些限制,并更准确地模拟成熟心肌细胞的特征。本文综述了近年来用于诱导心肌细胞成熟的多种方法的研究,特别强调了长链脂肪酸(LCFAs)的作用。本综述总结的证据来自利用新生小鼠或大鼠心肌细胞和hiPSC-CMs的研究。同时,未成熟心肌细胞已被证明主要依赖于糖酵解,通过成熟过渡到氧化磷酸化,从而增强电稳定性、收缩性和结构组织。LCFAs作为关键代谢因子,通过线粒体β-氧化产生ATP,从而提高代谢效率,在心肌细胞成熟过程中发挥关键作用。此外,LCFAs还参与激活细胞骨架成分和心肌细胞收缩性不可或缺的信号通路。重要的是,研究表明,当多种生化和生物物理刺激同时应用时,心肌细胞成熟的各个方面协同加速。因此,未来的研究重点是这些调节因子的协同应用,有望促进成熟过程,最终有助于生成适合再生医学和其他高级应用的成熟心肌细胞。本文章由计算机程序翻译,如有差异,请以英文原文为准。
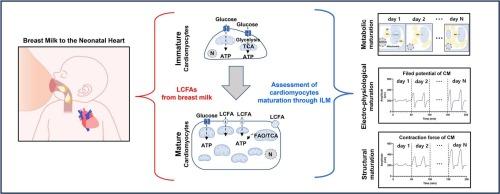
Metabolic impacts of long-chain fatty acids on cardiomyocyte maturation in neonatal mammalian hearts
Cardiomyocytes are essential models for cardiac disease modeling, drug development, and regenerative therapies. Specifically, human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) have emerged as widely used cellular models with high reproducibility. However, cardiomyocytes generated in vitro tend to remain immature and insufficient in replicating the electrophysiological and mechanical functions of adult cardiomyocytes, limiting the clinical and experimental applications of these models. Thus, various biochemical and biophysical strategies have been explored to promote the maturation of cardiomyocytes, to address these limitations, and more accurately mimic the characteristics of mature cardiomyocytes. This review summarizes recent studies on multiple methodologies employed to induce cardiomyocyte maturation, with a particular emphasis on the role of long-chain fatty acids (LCFAs). The evidence summarized in this review is derived from studies utilizing cardiomyocytes from neonatal mice or rats and hiPSC-CMs. Meanwhile, immature cardiomyocytes have been demonstrated to predominantly rely on glycolysis, transitioning to oxidative phosphorylation through maturation, which enhances electrical stability, contractility, and structural organization. LCFAs play a key role in the cardiomyocyte maturation process by serving as key metabolic factors that generate ATP through mitochondrial β-oxidation, thereby improving metabolic efficiency. Additionally, LCFAs are involved in activating cytoskeletal components and signaling pathways integral to cardiomyocyte contractility. Importantly, studies suggest that when multiple biochemical and biophysical stimuli are simultaneously applied, various aspects of cardiomyocyte maturation are synergistically accelerated. Therefore, future studies focusing on the coordinated application of these regulatory factors are expected to enhance the maturation process, ultimately contributing to the generation of mature cardiomyocytes suitable for regenerative medicine and other advanced applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Methods
生物-生化研究方法
CiteScore
9.80
自引率
2.10%
发文量
222
审稿时长
11.3 weeks
期刊介绍:
Methods focuses on rapidly developing techniques in the experimental biological and medical sciences.
Each topical issue, organized by a guest editor who is an expert in the area covered, consists solely of invited quality articles by specialist authors, many of them reviews. Issues are devoted to specific technical approaches with emphasis on clear detailed descriptions of protocols that allow them to be reproduced easily. The background information provided enables researchers to understand the principles underlying the methods; other helpful sections include comparisons of alternative methods giving the advantages and disadvantages of particular methods, guidance on avoiding potential pitfalls, and suggestions for troubleshooting.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


