α-五氟磺酰基酮C-C σ-键上芳香烃的插入:获得2-(五氟磺酰基)甲基二苯甲酮
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
由于其独特的物理化学性质和在药品和农用化学品中的潜在应用,将五氟磺胺基(SF5)纳入有机分子具有重要意义。然而,合成苯基sf5化合物的一般和有效的方法仍未被探索。本文报道了通过在α-五氟磺酰酮的C-C σ-键上插入芳香烃,合成了一系列2-(五氟磺酰)甲基二苯甲酮。21个例子证明,这种无过渡金属的反应在温和的条件下进行,具有高的区域选择性和良好的官能团耐受性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
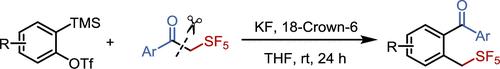
Insertion of Arynes into the C–C σ-Bond of α-Pentafluorosulfanyl Ketones: Access to 2-(Pentafluorosulfanyl)methyl Benzophenones
The incorporation of the pentafluorosulfanyl (SF5) group into organic molecules is highly significant due to its unique physicochemical properties and potential applications in pharmaceuticals and agrochemicals. However, general and efficient methods for the synthesis of benzylic SF5-containing compounds remain unexplored. Herein, we report the synthesis of a series of 2-(pentafluorosulfanyl)methyl benzophenones via the insertion of arynes into the C–C σ-bond of α-pentafluorosulfanyl ketones. This transition-metal-free reaction proceeds under mild conditions with high regioselectivity and good functional group tolerance, as demonstrated by 21 examples.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


