钴催化全氟烷基乙烯的抗马尔可夫尼科夫选择性氢硼化反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在DMAC中有Co(acac)2和dppf存在的情况下,建立了一种抗马氏选择性全氟烷基乙基硼酸酯的反马氏选择性全氟烷基乙基硼酸氢化反应简易方案。随后,全氟烷基乙基硼酸酯衍生物进一步转化为2-全氟烷基乙醇、酰胺和交叉偶联产品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
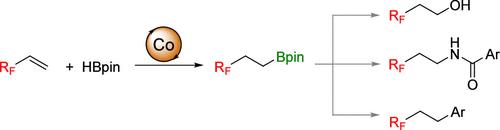
Cobalt-Catalyzed anti-Markovnikov-Selective Hydroborylation of Perfluoroalkylethylenes
A facile protocol for anti-Markovnikov-selective hydroborylation of perfluoroalkylethylenes with pinacolborane has been developed in the presence of Co(acac)2 and dppf in DMAC to furnish diverse perfluoroalkylethylboronate esters. Subsequently, perfluoroalkylethylboronate ester derivatives were further transformed into 2-perfluoroalkyl ethanol, amide, and cross-coupling products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


