可定制丝素蛋白为基础的水凝胶纤维支架按需多方面组织修复
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
水凝胶支架是一种有吸引力的组织修复工具。然而,靶向组织修复需要特定的形状和生物功能设计,大多数天然蛋白基水凝胶支架主要局限于特定的组织修复应用。在此,我们通过协同静电纺丝、光聚合和金属配位策略开发了一个多功能的结构仿生天然蛋白质平台。通过将甲基丙烯酸丝素(SFMA)与丙烯酸双膦酸盐(AcBP)相结合,我们开发了一种动态功能化的基质,它可以实现(1)通过可调静电纺丝收集器实现可定制的形状控制,(2)通过金属离子螯合实现按需生物功能定制。作为概念的证明,我们展示了该平台的场景特异性治疗效果:(i) Mg2+功能化膜(S-LB-Mg)在临界尺寸的头颅缺陷中协调血管生成-成骨耦合,(ii) Ag+集成填充物(S-LB-Ag)通过非抗生素机制实现细菌根除并加速感染伤口愈合,以及(iii) Zn2+负载导管(S-LB-Zn)驱动巨噬细胞M2极化以增强周围神经再生。这种天然衍生的基于蛋白质的平台克服了与临床生物活性因子/抗生素复合支架相关的潜在副作用,以经济高效的方式为多种组织的修复和再生提供了一种简单而可定制的解决方案。总的来说,我们的策略为构建具有可定制功能和形状的蛋白质衍生水凝胶微纤维提供了另一种视角,用于组织修复应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
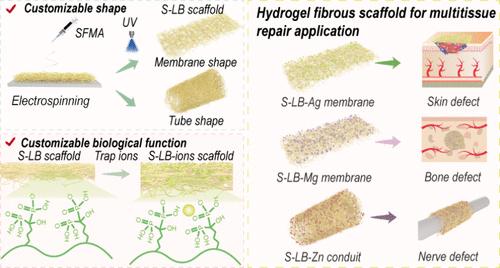
Customizable Silk Fibroin-Based Hydrogel Fibrous Scaffold for On-Demand Multifaceted Tissue Repair
Hydrogel scaffolds represent an attractive tool for tissue repair. However, targeted tissue repair requires a specific shape and biological function design, and most natural-protein-based hydrogel scaffolds are predominantly confined to specific tissue repair applications. Here, we developed a versatile structural biomimetic natural protein platform through synergistic electrospinning, photopolymerization, and metal-coordination strategies. By integrating methacrylated silk fibroin (SFMA) with acrylated bisphosphonates (AcBP), we developed a dynamically functionalizable matrix that enables (1) customizable shape control via tunable electrospinning collectors and (2) on-demand biological function customization through metal-ion chelation. As a proof of concept, we demonstrate this platform’s scenario-specific therapeutic efficacy: (i) Mg2+-functionalized membranes (S-LB-Mg) that orchestrate angiogenic-osteogenic coupling in critical-sized calvarial defects, (ii) Ag+-integrated dressing (S-LB-Ag) enabling bacterial eradication via a nonantibiotic mechanism and accelerating infected wound closure, and (iii) Zn2+-loaded conduits (S-LB-Zn) that drive macrophage M2 polarization to enhance peripheral nerve regeneration. This naturally derived protein-based platform overcomes the potential side effects associated with clinical bioactive factor/antibiotic composite scaffolds, offering a simple and customizable solution for the repair and regeneration of diverse tissues in a cost-effective yet highly effective manner. Overall, our strategy provides an alternative perspective for constructing protein-derived hydrogel microfibers with customizable functions and shapes for tissue repair applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


