强酸中铵的低频超声高效电化学氧化:铵的脱溶作用
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
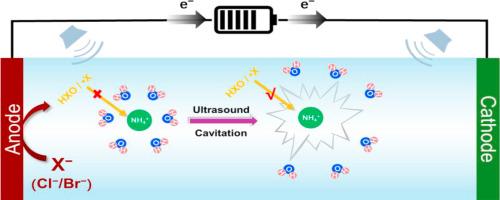
电化学高级氧化法(EAOP)利用活性卤素处理富含NH4+的废水是一种有前途的替代生物方法,但由于NH4+氧化动力学差,在强酸环境中面临挑战。在此,我们确定NH4+的强溶剂化是关键的限制因素,其中周围的H2O分子形成了阻碍氧化物种的保护屏障。为了解决这个问题,引入了低频超声(LFS,例如40 kHz),通过空化破坏溶剂化环境并增加NH4+对氧化物质的暴露。在pH为2时,单独电解2.5 h内NH4+的氧化效率仅为8.0 ~ 28.8%,而在LFS-Cl/EAOP体系中,该值显著提高(60.73 ~ 100%),在LFS-Br/EAOP体系中达到100%。LFS-Cl/EAOP体系对负极材料有很强的依赖性,掺硼金刚石(BDD)和PbO2的负极性能优于混合金属氧化物(MMO)和Pt;相比之下,无论阳极类型如何,LFS-Br/EAOP体系都能获得较高的NH4+去除率。进一步揭示了溴酸盐的形成受到HBrO快速消耗和阴极还原的抑制。此外,还证明了LFS-Br/EAOP完全去除NH4+和酸溶液再生吸附NH3的可行性,突出了其实际应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Highly efficient electrochemical oxidation of ammonium in strong acid via low-frequency sonication: The role of ammonium desolvation
The use of electrochemical advanced oxidation process (EAOP) for treating NH4+-rich wastewater via reactive halogen species offers a promising alternative to biological methods but faces challenges in strong acid due to poor NH4+ oxidation kinetics. Herein, we identify the strong solvation of NH4+ as the key limiting factor, where surrounding H2O molecules form a protective shield that hinders oxidative species. To address this, low-frequency sonication (LFS, e.g., 40 kHz) was introduced, disrupting the solvation environment through cavitation and enhancing NH4+ exposure to oxidative species. At pH 2, electrolysis alone exhibited only 8.0–28.8 % NH4+ oxidation efficiency within 2.5 h, while this value significantly improved (60.73–100 %) in the LFS-Cl/EAOP systems and reached 100 % in the LFS-Br/EAOP systems. The LFS-Cl/EAOP systems showed strong dependence on anode materials, with boron-doped diamond (BDD) and PbO2 outperforming mixed metal oxides (MMO) and Pt; in contrast, the LFS-Br/EAOP systems achieved high NH4+ removal regardless of anode type. It is further revealed that bromate formation was suppressed by rapid HBrO consumption and cathodic reduction. Moreover, the feasibility of complete NH4+elimination and acid solution regeneration for NH3 adsorption via LFS-Br/EAOP was demonstrated, highlighting its potential for practical application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


