面向企业范围的制药4.0采用
IF 3.3
Q2 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
工业4.0在制药行业的采用被称为制药4.0。从运营效率到法规遵从性,制药4.0为制药组织带来了许多显著的好处。本研究评估了文献中现有的制药4.0采用框架,旨在开发一个结构化和适应性强的制药4.0企业级采用概念框架。我们进行了一项系统评价,以识别和分析现有的Pharma 4.0框架。所研究的框架被分类为制造和质量管理、供应链管理、临床试验和研发数字化、劳动力能力和制药5.0。对已确定框架的分析表明,虽然它们提供了数字化转型战略,但它们只关注特定领域。为此,本文提出了一个概念性的制药4.0框架,强调组织准备评估、核心技术集成、网络安全和数据管理、系统集成和持续改进。该框架为制药4.0的采用提供了实用的路线图,提高了法规遵从性和运营效率。此外,该框架还支持各种各样的制药组织,包括非洲和其他发展中国家的中小型企业。因此,本研究为企业范围内的制药4.0采用提供了一个新的结构化框架,并通过建立关键成功因素来解决其关键实施障碍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
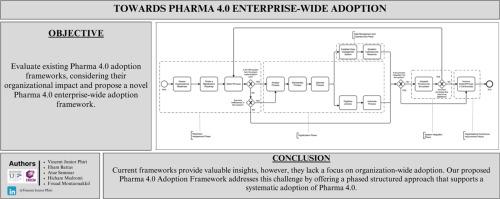
Towards enterprise-wide pharma 4.0 adoption
The adoption of Industry 4.0 in the pharmaceutical industry is referred to as Pharma 4.0. Pharma 4.0 procures pharmaceutical organizations many significant benefits ranging from operational efficiency to regulatory compliance. This study evaluates existing Pharma 4.0 adoption frameworks in literature and aims to develop a structured and adaptable Pharma 4.0 enterprise-wide adoption conceptual framework. A systematic review was conducted to identify and analyze existing Pharma 4.0 frameworks. The examined frameworks were categorized into Manufacturing and Quality Management, Supply Chain Management, Clinical Trials and R&D Digitalization, Workforce Competence, and Pharma 5.0. Analysis of the identified frameworks reveals that while they offer strategies for digital transformation, they focus solely on specific areas. In response, a conceptual Pharma 4.0 framework is proposed, emphasizing organizational readiness assessment, core technology integration, cybersecurity and data management, system integration, and continuous improvement. This framework provides a practical roadmap for Pharma 4.0 adoption, improving regulatory compliance and operational efficiency. Also, the framework supports a diverse range of pharmaceutical organizations, including small and medium-sized enterprises (SMEs) in Africa and other developing nations. Thus, this study contributes a novel structured framework for enterprise-wide Pharma 4.0 adoption and addresses its key implementation barriers by establishing critical success factors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Scientific African
Multidisciplinary-Multidisciplinary
CiteScore
5.60
自引率
3.40%
发文量
332
审稿时长
10 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


