以黏液酸为原料合成不饱和1,6-二酮和1,6-二醇
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
顺式、顺式mucic酸(HO2C-CH = ch = ch = CH-CO2H)与苯基超强酸TfOH中的芳烃反应,生成芳香酰化产物,即不饱和的E-,E-1,6-二酮[ArOC-CH = ch = CH-COAr]。这些二酮也可在芳烃的丙烯酰化反应中得到。TfOH中的反应是通过中间形成的O,O-二质子化形式的黏液酸[HO(+HO = C) -CH = ch = CH-CH = CH-C (= OH)+OH]进行的,该反应通过NMR实验和DFT计算进行了理论研究。这些1,6-二酮用NaBH4羰基还原生成相应的不饱和非对映异构体E-,E-1,6-二醇[Ar(HO) CH-CH = CH-CH = CH-CH (OH)Ar]。1,6-二酮的碳-碳键与碳上的Pd或Pt催化加氢得到相应的1,6-二酮(ArOC-CH2CH2CH2CH2-COAr)。研究的反应有助于以工业化学中重要的化合物粘液酸为基础的有机合成的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
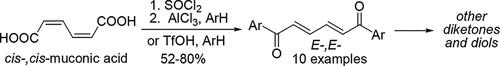
Synthesis of Unsaturated 1,6-Diketones and 1,6-Diols from Muconic Acid
Cis-,cis-muconic acid (HO2C–CH═CH–CH═CH–CO2H) reacts with arenes in Bro̷nsted superacid TfOH, affording the aromatic acylation products, unsaturated E-,E-1,6-diketones [ArOC–CH═CH–CH═CH–COAr]. These diketones are also obtained in Friedel–Crafts acylation of arenes by muconoyl chloride. The reaction in TfOH proceeds through an intermediate formation of muconic acid O,O-diprotonated form [HO(+HO═C)–CH═CH–CH═CH–C(═OH)+OH], which was investigated experimentally by NMR and theoretically by DFT calculations. Carbonyl reduction of these 1,6-diketones with NaBH4 results in the formation of the corresponding unsaturated diastereomeric E-,E-1,6-diols [Ar(HO)CH–CH═CH–CH═CH–CH(OH)Ar]. Catalytic hydrogenation of carbon–carbon bonds in 1,6-diketones with Pd or Pt on carbon furnishes the corresponding 1,6-diketones (ArOC–CH2CH2CH2CH2–COAr). The studied reactions contribute to the development of organic synthesis on the basis of transformations of muconic acid, which is an important compound in industrial chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


