腐植酸介导的新途径促进晶体铁矿氧化过程中远距离羟基自由基的产生
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
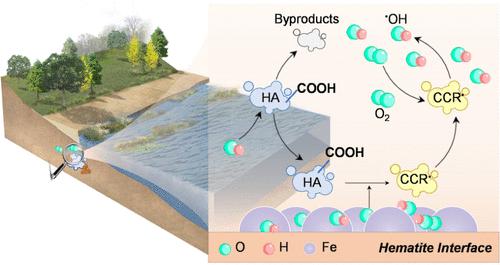
羟基自由基(•OH)是由晶体铁矿物在氧化还原振荡下广泛产生的,在此过程中,它们对污染物动力学的氧化影响往往受到低运输的限制。虽然天然有机物作为一种普遍存在的氧化还原介质存在,但它如何调节异构系统中•OH生成的距离尺度仍然知之甚少。本研究首次报道了腐植酸(HA)介导的一种未知途径,可扩大还原赤铁矿(rHem)氧化后•OH的空间范围。结果表明,透明质酸促进了•OH从表面到溶液的空间再分布,对不同表面可达性的污染物的氧化效果产生相反的影响。很难用醌类或溶解的Fe-HA配合物的电子穿梭来解释自由•OH生成的增强。综合证据表明,吸附的HA优先消耗表面结合的•OH,引发碳中心自由基(CCR•)的固-液传播,主要促进远离表面的二次游离•OH的形成。反应输运模拟和原位荧光结果显示了CCR•介导的界面和厘米尺度的远距离OH生成。傅里叶变换离子回旋共振质谱法验证了吸附HA的表面脱羧作用在CCR•生成中的作用。这些发现为铁氧化还原循环在自然和工程系统(包括天然有机物)中产生的•OH的环境命运提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
New Pathway Mediated by Humic Acid Facilitates Long-Distance Hydroxyl Radical Production during Crystalline Iron Mineral Oxygenation
Hydroxyl radicals (•OH) are extensively produced from crystalline iron minerals under redox oscillation, during which their oxidizing impact on pollutant dynamics is often limited by low transportation. While natural organic matter exists as a ubiquitous redox mediator, how it regulates the distance scale of •OH production in a heterogeneous system remains poorly understood. This study, for the first time, reports an unrecognized route mediated by humic acid (HA) in extending the spatial range of •OH from oxygenation of reduced hematite (rHem). The results showed that HA facilitated the spatial redistribution of •OH from the surface to the solution, posing inverse impacts on oxidation efficacies of pollutants with varying surface accessibilities. It was difficult to explain the enhanced free •OH production using electron shuttling by quinones or dissolved Fe–HA complexes. Comprehensive evidence demonstrated that adsorbed HA preferentially consumed surface-bound •OH to trigger a solid-to-liquid propagation of carbon-centered radicals (CCR•), dominantly promoting formation of secondary free •OH far from the surface. Reactive-transport simulation and in situ fluorescence results visualized interfacial and centimeter-scale long-range production of •OH mediated by CCR•. Fourier transform ion cyclotron resonance mass spectrometry verified the role of surface decarboxylation of adsorbed HA in CCR• generation. These findings offer new insights into the environmental fate of •OH produced during iron redox cycling in natural and engineered systems, including those with natural organic matter.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


