利用生物矿化病毒样颗粒的增强型纳米疫苗通过鼻到脑传递途径对胶质母细胞瘤进行有效免疫治疗
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
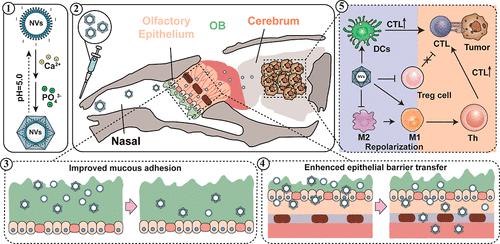
神经胶质瘤是一种中枢神经系统的原发性恶性肿瘤,尽管在治疗方面取得了进展,但它仍然是一个巨大的挑战。免疫疗法有希望,但其疗效受到血脑屏障(BBB)和免疫抑制肿瘤微环境(TME)的阻碍。鼻到脑的运输途径为绕过血脑屏障和避免全身吸收问题提供了一个潜在的解决方案。在这项研究中,我们开发了一种基于乙型肝炎核心抗原衍生病毒样颗粒(HBc VLPs)的磷酸钙(CaP)覆盖纳米疫苗(NV),优化了粘膜递送应用。生物矿化后,NVs在胶质瘤组织中表现出增强的粘膜粘连和显著增加的积聚。此外,通过利用HBc VLPs的免疫原性,并在其表面显示胶质瘤相关抗原EphA2671-679,这种NVs既可以作为免疫原,也可以作为佐剂。它们通过增加有效的T细胞浸润,减少调节性T细胞和m2型肿瘤相关巨噬细胞,促进了显著的抗胶质瘤治疗效果,并引发了强大、持久的肿瘤抑制。这种创新的NV构建策略突出了cap包被的vlp在增强鼻到脑传递和免疫增强方面的潜力,为开发有效的神经胶质瘤免疫疗法提供了一条有希望的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced Nano-Vaccine Utilizing Biomineralized Virus-like Particles for Efficient Glioblastoma Immunotherapy via the Nose-To-Brain Delivery Pathway
Glioma, a primary malignant tumor of the central nervous system, remains a formidable challenge despite advancements in treatment. Immunotherapies hold promise, but their efficacy is hindered by the blood–brain barrier (BBB) and immunosuppressive tumor microenvironment (TME). The nose-to-brain transport pathway offers a potential solution for bypassing the BBB and avoiding systemic absorption issues. In this study, we developed a calcium phosphate (CaP)-covered nanovaccine (NV) based on hepatitis B core antigen derived virus-like particles (HBc VLPs), optimized for mucosal delivery applications. After biomineralization, this NVs exhibited enhanced mucosal adhesion and significantly higher accumulation in glioma tissue. Furthermore, by leveraging the immunogenicity of HBc VLPs and displaying a glioma-associated antigen EphA2671–679 on its surface, this NVs served as both immunogen and adjuvant. They promoted significant antiglioma therapeutic efficacy and elicited robust, durable tumor suppression by increasing effective T cell infiltration, reducing regulatory T cells and M2-type tumor-associated macrophages. This innovative NV construction strategy highlights the potential of CaP-coated VLPs for enhancing nose-to-brain delivery and immunological enhancement, offering a promising avenue for the development of effective immunotherapies for glioma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


