发现CYP1A1抑制剂用于败血症的宿主定向治疗
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
细菌性败血症仍然是全球死亡的主要原因,多药耐药(MDR)的增加加剧了这一情况。宿主定向治疗(HDT)已成为一种有前途的非抗生素治疗感染的方法;因此,已经确定了多个HDT目标。然而,将HDT靶点转化为治疗药物,特别是小分子药物,仍然很少。我们的研究重点是细胞色素P4501A1 (CYP1A1),宿主抗感染能力的负调节因子。通过深度学习、虚拟筛选和生物学评估,我们发现了CYP1A1的新型小分子抑制剂。经过结构优化,化合物38和47表现出优异的活性,通过增强巨噬细胞吞噬作用,使耐甲氧西林金黄色葡萄球菌(MRSA)和鲍曼不动杆菌的细菌负荷降低70%以上。这项工作强调了CYP1A1作为一个有价值的HDT靶点,并表明用一个单一的小分子化合物抑制它可以为治疗耐多药细菌诱导的脓毒症提供一个潜在的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
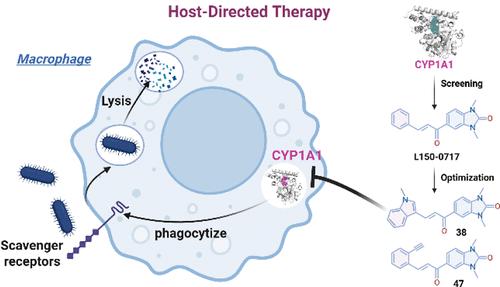
Discovery of CYP1A1 Inhibitors for Host-Directed Therapy against Sepsis
Bacterial sepsis remains a leading cause of death globally, exacerbated by the rise of multidrug resistance (MDR). Host-directed therapy (HDT) has emerged as a promising nonantibiotic approach to combat infections; thus, multiple HDT targets have been identified. However, the translation of HDT targets into therapeutic drugs, particularly small-molecule drugs, remains rare. Our study focuses on cytochrome P4501A1 (CYP1A1), a negative regulator of host antiinfection capabilities. Using deep learning, virtual screening, and biological evaluation, we identified novel small-molecule inhibitors of CYP1A1. After structural optimization, compounds 38 and 47 demonstrated exceptional activity, reducing bacterial loads of methicillin-resistant Staphylococcus aureus (MRSA) and Acinetobacter baumannii by over 70% by enhancing macrophage phagocytosis. This work highlights CYP1A1 as a valuable HDT target and shows that inhibiting it with a single small-molecule compound can offer a potential solution to treat MDR bacterial-induced sepsis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


