新一代TRK抑制剂抗获得性耐药突变治疗实体瘤的结构优化
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
第一代TRK抑制剂已有效应用于临床肿瘤治疗。然而,获得性耐药经常发生,主要归因于耐药TRK突变,特别是普遍存在的xDFG TRKAG667C突变。在此,我们通过利用构象限制策略,从我们团队先前发现的先导化合物7开始,揭示了新一代TRK抑制剂的设计。其中,化合物100对Ba/ f3 - mip - trkag667c细胞系的抗增殖活性优于selitrectinib(最先进的选择性下一代TRK抑制剂之一),具有高选择性的抑制TRK激酶活性。此外,100·HCl在小鼠体内表现出良好的药代动力学特征和良好的口服生物利用度。在Ba/F3-MPRIP-TRKAG667C皮下肿瘤模型中,100·HCl体内处理导致肿瘤生长明显延迟。因此,我们的工作为开发下一代TRK抑制剂提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
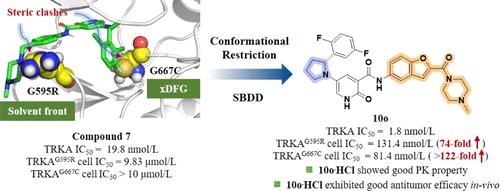
Structural Optimization of Next-Generation TRK Inhibitors against Acquired Drug Resistance Mutations for the Treatment of Solid Tumors
First-generation TRK inhibitors have been effectively employed in clinical oncology treatments. However, acquired resistance frequently develops, primarily attributed to resistant TRK mutants, particularly the prevalent xDFG TRKAG667C mutation. Herein, we unveil the design of novel next-generation TRK inhibitors by leveraging a conformational restriction strategy, beginning with lead compound 7, which was previously discovered by our team. Among them, compound 10o exhibited superior antiproliferative activity in the Ba/F3-MPRIP-TRKAG667C cell line compared to selitrectinib, one of the most advanced selective next-generation TRK inhibitors, and it potently inhibited TRK kinase activity with high selectivity. Furthermore, 10o·HCl showed promising pharmacokinetic profiles with good oral bioavailability in mice. In vivo treatment with 10o·HCl led to a marked delay in tumor growth in a Ba/F3-MPRIP-TRKAG667C subcutaneous tumor model. Thus, our work offers valuable insights for the development of next-generation TRK inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


