废电池中降解LiCoO2的再生:高效酸浸和结构改性以增强能量储存
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
再生LiCoO2的电化学性能对其利用具有重要意义。本文在废锂离子电池再生过程中对LiCoO2的结构进行了控制。研究了再生LiCoO2的结构调整机理。讨论了LiCoO2与其电化学性能的关系。在浸出过程中考虑了Co2+/Li+离子的溶解能力和渗滤液的最终pH。用NaOH沉淀Co2+离子,并在低温(400-500℃)下煅烧沉淀,加速Co3O4(111)面。这有利于LiCoO2(001)平面的形成和电化学性能的提高。优化后的LiCoO2在0.5℃下的初始容量为153.4 mA h·g-1,循环150次后容量保持率为89.0%。Li+/Co2+离子浓度达到6.6/54.8 g·L-1,最终pH为2.18。这项工作为制备高性能的再生LiCoO2提供了一种有效的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
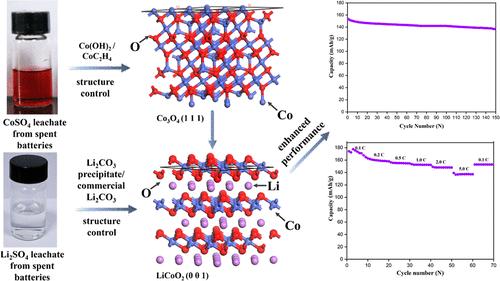
Regeneration of Degraded LiCoO2 from Spent Batteries: Efficient Acid Leaching and Structure Modification for Enhanced Energy Storage
Electrochemical performance of regenerated LiCoO2 is important for its utilization. Herein, the structure of LiCoO2 was controlled during its regeneration from spent lithium-ion batteries. The mechanism for adjusting the structure of regenerated LiCoO2 is investigated. The relationship between LiCoO2 and its electrochemical performance is discussed. The dissolving capacity of Co2+/Li+ ions and the final pH of the leachate are considered during the leaching process. The (111) plane of Co3O4 is accelerated by precipitating Co2+ ions with NaOH and calcining the precipitate at a low temperature (400–500 °C). It is beneficial for developing the (001) plane of LiCoO2 and improves its electrochemical performance. The optimized LiCoO2 achieves an initial capacity of 153.4 mA h·g–1 at 0.5 C with 89.0% capacity retention after 150 cycles. The concentrations of Li+/Co2+ ions reach 6.6/54.8 g·L–1, and the final pH is 2.18. This work provides an effective strategy to prepare regenerated LiCoO2 with a high performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


