多巴胺能传递模式的编码原理。
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
多巴胺能神经元通过不同的放电模式影响不同的行为,但确切的机制尚不清楚。我们引入了一种基于多路遗传编码传感器的成像和伏安法,同时记录小鼠中枢神经元突触、突触周围和突触外的多巴胺能传递。使用这种方法和基于基因编码传感器的图像分析程序,我们发现异质多巴胺能发射模式创造了不同的传输模式,编码频率,数量和发射脉冲的同步性,使用神经递质数量,释放突触计数,突触和/或体积传输。在强直期和低频期活动下,转运体在突触周围有效地再摄取多巴胺,将多巴胺限制在突触间隙内以介导突触传递。相反,在高频,特别是同步放电活动或转运蛋白抑制下,释放的多巴胺可能压倒转运蛋白,通过一到三个出口通道从突触间隙逃逸,触发体积传递。我们的研究阐明了突触外壳、特性和转运体的协作机制,这些机制定义了活动模式依赖的多巴胺能传递模式的编码原则。本文章由计算机程序翻译,如有差异,请以英文原文为准。
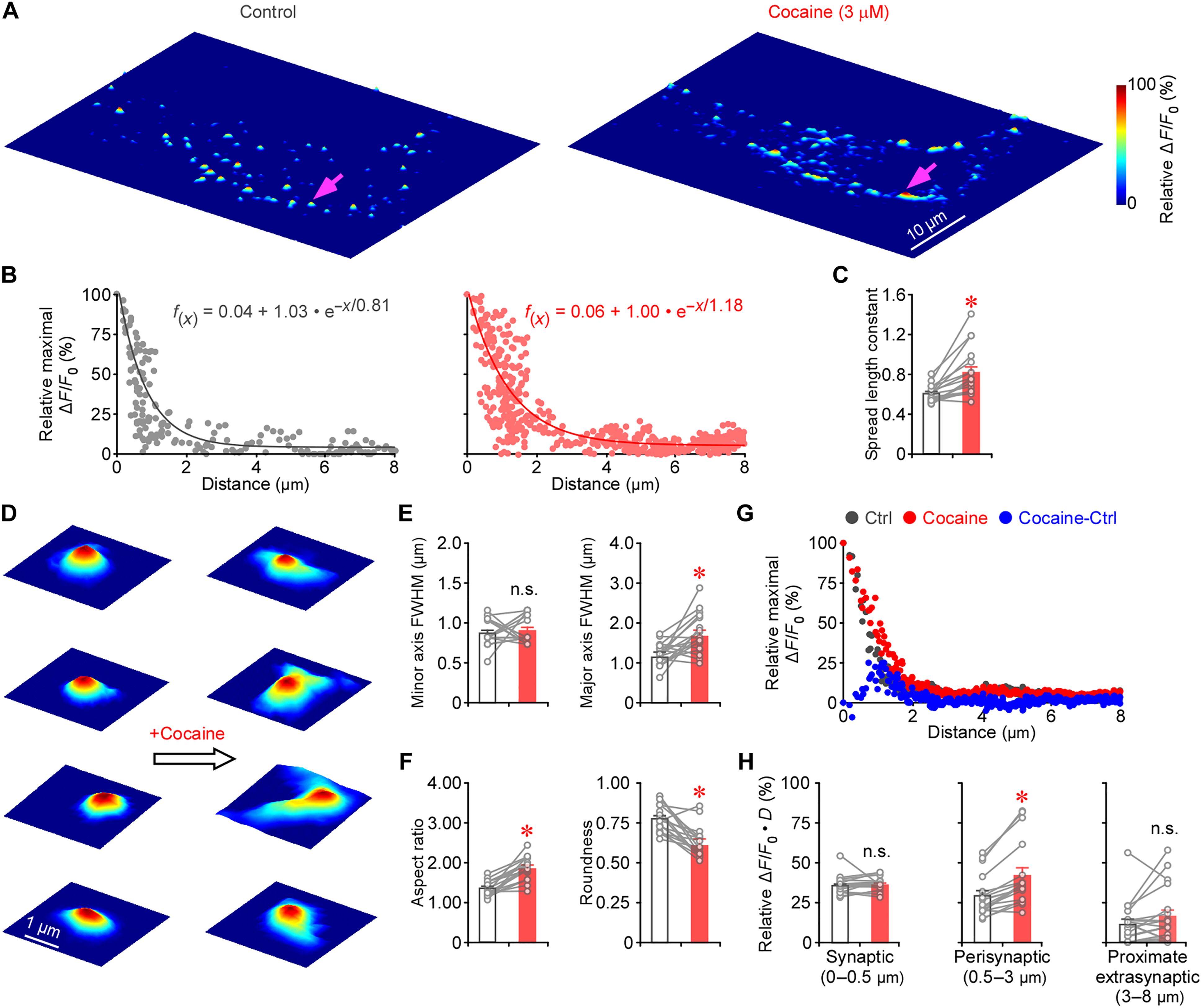
Coding principles of dopaminergic transmission modes
Dopaminergic neurons influence diverse behaviors with varied firing patterns, yet the precise mechanisms remain unclear. We introduce a multiplexed genetically encoded sensor–based imaging and voltammetry method to simultaneously record synaptic, perisynaptic, and extrasynaptic dopaminergic transmission at mouse central neurons. Using this method alongside a genetically encoded sensor–based image analysis program, we found that heterogeneous dopaminergic firing patterns create various transmission modes, encoding frequency, number, and synchrony of firing pulses using neurotransmitter quantity, releasing synapse count, and synaptic and/or volume transmission. Under both tonic and low-frequency phasic activities, transporters effectively reuptake dopamine at perisynaptic sites, confining dopamine within synaptic clefts to mediate synaptic transmission. In contrast, under high-frequency, particularly synchronized firing activity or transporter inhibition, released dopamine may overwhelm transporters, escaping from synaptic clefts via one to three outlet channels, triggering volume transmission. Our study illuminates a collaborative mechanism of synaptic enclosures, properties, and transporters that defines the coding principles of activity pattern–dependent dopaminergic transmission modes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


