用于癌症治疗的天然抗癌螯合剂的第一排过渡金属配合物
IF 23.5
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
金属配合物中的配体是决定配合物治疗效果的关键。这种活性调节是多方面的,受亲脂性增强、氧化还原和光谱特性改变、细胞摄取、溶解度和细胞毒性协同增强等因素的影响。从钒(V)到铜(Cu)的第一行生物必需过渡金属配合物,以其可变氧化态、有趣的氧化还原性质和生物必需性质为特征,为开发具有内在抗癌特性的有机配体的下一代金属配合物提供了有希望的途径,特别是那些来自天然来源的金属配合物。本文综述了含有五种具有螯合性质的天然抗癌配体的第一行过渡金属配合物(V - Zn)的最新进展,即姜黄素、类黄酮、香豆素、萘醌和次氯酸酯。姜黄素本身含有一个O,O给体双齿配位位点,而其他四类化合物的天然衍生物因其固有的双齿O,O给体位点而被选中。这些配位位点通常由两个相邻羟基(OH)基团(香豆素)的两个氧原子组成,或者一个羰基(C=O)基团的氧原子和另一个羟基(OH)基团的氧原子组成(对于类黄酮、萘醌和邻苯二甲酸乙酯),以确保络合形成的螯合环具有足够的热力学稳定性。强调我们的实验室和其他研究人员在这个新兴领域的发现,我们探索这些复合物在抗癌应用中的潜力。此外,我们探索了涉及第一排过渡金属配合物与天然光活性配体偶联的研究,作为癌症光疗的有效光敏剂。最后,我们讨论了这一重要研究领域的前景和挑战。我们相信这种及时的汇编将通过提供对最近和最新发现的见解并促进这一新兴领域的进一步发展而使研究人员受益。本文章由计算机程序翻译,如有差异,请以英文原文为准。
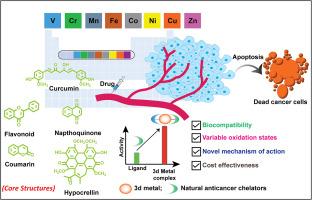

First-row transition metal complexes of naturally occurring anticancer chelators for cancer treatment
Ligands in a metal complex are crucial in determining the therapeutic effectiveness of the complex. This activity modulation is multifaceted, influenced by factors such as enhanced lipophilicity, alterations in redox and spectroscopic properties, cellular uptake, solubility, and synergistic enhancement of cytotoxicity. Complexes of first-row bioessential transition metals from vanadium (V) to copper (Cu), characterized by their variable oxidation states, intriguing redox properties, and bioessential nature, present promising avenues for developing next-generation metal complexes with organic ligands possessing intrinsic anticancer properties, particularly those derived from natural sources. This review article explores recent and up-to-date advancements in first-row transition metal complexes (V to Zn) incorporating five distinct types of naturally occurring anticancer ligands endowed with chelating properties, namely, curcumin, flavonoids, coumarin, naphthoquinone, and hypocrellins. Curcumin inherently contains an O,O-donor bidentate coordination site, while natural derivatives from the other four classes of compounds were selected for their intrinsic bidentate O,O-donor sites. These coordination sites typically consist of either two oxygen atoms from two adjacent hydroxyl (OH) groups (coumarin) or one oxygen atom from a carbonyl (C=O) group and another from a hydroxyl (OH) group (for flavonoids, naphthoquinone, and hypocrellins), ensuring sufficient thermodynamic stability of the chelate rings formed upon complexation. Emphasizing findings from our laboratory and other investigators in this burgeoning field, we explore the potential of these complexes in anticancer applications. Additionally, we explore studies involving first-row transition metal complexes coupled with naturally occurring photoactive ligands, serving as potent photosensitizers for cancer phototherapy. We conclude by addressing the prospects and challenges in this vital research area. We believe this timely compilation will benefit researchers by providing insights into recent and up-to-date findings and fostering further advancements in this emerging area.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Coordination Chemistry Reviews
化学-无机化学与核化学
CiteScore
34.30
自引率
5.30%
发文量
457
审稿时长
54 days
期刊介绍:
Coordination Chemistry Reviews offers rapid publication of review articles on current and significant topics in coordination chemistry, encompassing organometallic, supramolecular, theoretical, and bioinorganic chemistry. It also covers catalysis, materials chemistry, and metal-organic frameworks from a coordination chemistry perspective. Reviews summarize recent developments or discuss specific techniques, welcoming contributions from both established and emerging researchers.
The journal releases special issues on timely subjects, including those featuring contributions from specific regions or conferences. Occasional full-length book articles are also featured. Additionally, special volumes cover annual reviews of main group chemistry, transition metal group chemistry, and organometallic chemistry. These comprehensive reviews are vital resources for those engaged in coordination chemistry, further establishing Coordination Chemistry Reviews as a hub for insightful surveys in inorganic and physical inorganic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


