白细胞介素增强car工程免疫细胞在肿瘤免疫治疗中的应用:目前的见解和未来的展望
IF 3.7
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
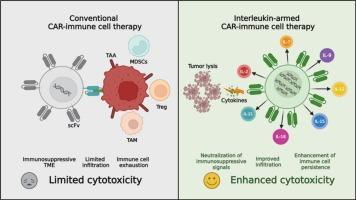
尽管嵌合抗原受体(CAR)-T细胞治疗血液系统恶性肿瘤取得了显著的临床成功,但传统的第二代CAR-T细胞治疗实体肿瘤的疗效仍然不理想,主要是由于三个主要的生物学障碍:(1)免疫抑制肿瘤微环境(TME),(2)肿瘤浸润能力不足,(3)T细胞衰竭机制。为了克服这些限制,创新的第四代“装甲”CAR-T细胞平台被设计成集成的细胞因子分泌模块,旨在通过局部免疫调节增强抗肿瘤反应。这些先进的细胞疗法实现了各种免疫刺激细胞因子直接在TME内的靶向递送,从而协调了三个关键的治疗效果:(1)免疫抑制生态位的重塑,(2)增强免疫细胞的持久性,(3)免疫抑制信号网络的中和。这篇全面的综述系统地检查了细胞因子分泌car工程免疫细胞的翻译应用,包括CAR-T、CAR-NK和CAR-iNKT细胞平台,在实体肿瘤免疫治疗中,特别强调了多种类型的免疫调节细胞因子,它们可以增强细胞毒性潜力,促进免疫细胞存活,并抵消tme介导的免疫抑制。我们批判性地评估了临床前和临床证据,证明了细胞因子修饰的car工程细胞在各种肿瘤模型中的治疗效果,包括血液病恶性肿瘤、胶质母细胞瘤和神经母细胞瘤。此外,本综述还讨论了当前的翻译挑战,特别是细胞因子相关的毒性特征和实现细胞因子释放时空控制的创新策略,同时讨论了它们对推进实体瘤免疫治疗临床结果的潜在影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Interleukin-enhanced CAR-engineered immune cells in tumor immunotherapy: current insights and future perspectives
Despite the remarkable clinical success of chimeric antigen receptor (CAR)-T cell therapy in hematologic malignancies, the therapeutic efficacy of conventional second-generation CAR-T cells in treating solid tumors remains suboptimal, primarily due to three major biological barriers: (1) the immunosuppressive tumor microenvironment (TME), (2) inadequate tumor infiltration capacity, and (3) T cell exhaustion mechanisms. To overcome these limitations, innovative fourth-generation “armored” CAR-T cell platforms have been engineered with integrated cytokine-secreting modules designed to potentiate anti-tumor responses through localized immunomodulation. These advanced cellular therapeutics achieve targeted delivery of various immunostimulatory cytokines directly within the TME, thereby orchestrating three critical therapeutic effects: (I) remodeling of the immunosuppressive niche, (II) enhancement of immune cell persistence, and (III) neutralization of immunosuppressive signaling networks. This comprehensive review systematically examines the translational applications of cytokine-secreting CAR-engineered immune cells, including CAR-T, CAR-NK, and CAR-iNKT cell platforms, in solid tumor immunotherapy, with particular emphasis on multiple classes of immunomodulatory cytokines that enhance cytotoxic potential, promote immune cell survival, and counteract TME-mediated immunosuppression. We critically evaluate preclinical and clinical evidence demonstrating the therapeutic efficacy of cytokine-armed CAR-engineered cells across various tumor models, including hematological malignancies, glioblastoma, and neuroblastoma. Furthermore, this review addresses current translational challenges, particularly cytokine-associated toxicity profiles and innovative strategies for achieving spatiotemporal control of cytokine release, while discussing their potential implications for advancing clinical outcomes in solid tumor immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cytokine
医学-免疫学
CiteScore
7.60
自引率
2.60%
发文量
262
审稿时长
48 days
期刊介绍:
The journal Cytokine has an open access mirror journal Cytokine: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
* Devoted exclusively to the study of the molecular biology, genetics, biochemistry, immunology, genome-wide association studies, pathobiology, diagnostic and clinical applications of all known interleukins, hematopoietic factors, growth factors, cytotoxins, interferons, new cytokines, and chemokines, Cytokine provides comprehensive coverage of cytokines and their mechanisms of actions, 12 times a year by publishing original high quality refereed scientific papers from prominent investigators in both the academic and industrial sectors.
We will publish 3 major types of manuscripts:
1) Original manuscripts describing research results.
2) Basic and clinical reviews describing cytokine actions and regulation.
3) Short commentaries/perspectives on recently published aspects of cytokines, pathogenesis and clinical results.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


