一种新型、绿色的UFLC-MS/MS定量纳米聚合物中金刚烷胺和左旋多巴的方法:用于测定载药量(%DL)、包裹度(%DEE)和药物释放谱
IF 3.7
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
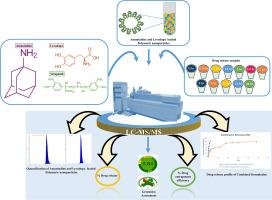
左旋多巴是一种多巴胺前体,是帕金森病治疗中广泛使用的处方药,它具有左旋多巴诱导的运动障碍(LID)的副作用。盐酸金刚烷胺是一种NMDA受体拮抗剂,与左旋多巴共包被,形成聚合物纳米颗粒(NPs),以克服副作用并协同作用,提高治疗效果。在目前的工作中,我们开发了一种新型的绿色超快速液相色谱-串联质谱(UFLC-MS/MS)技术,用于同时定量聚合物纳米颗粒中的金刚烷胺和左旋多巴。具有多反应监测(MRM)扫描模式和大气压化学电离(APCI)源的三重四极杆分析仪。色谱柱为Waters Symmetry C8柱(150 × 4.6 mm, 3.5 μm),保持温度为40℃。为确保分析物检测的敏感性和特异性,流动相为0.1%甲酸水溶液和甲醇(40:60),总运行时间为5 min。通过方法验证验证了良好的线性、回收率、准确性和灵敏度。绿色评估采用AGREE、GAPI和AES指标。结果建立的绿色UFLC-MS/MS方法能有效地定量左旋多巴和金刚烷胺在聚合物纳米颗粒中的含量,并能准确评价其%DL、%DEE和药物释放谱。左旋多巴(89.6%)和金刚烷胺(90.16%)的DEE值为%,相应的DL值分别为20.5%和24.10%,说明其载药量较大。体外释药表现为缓释行为,左旋多巴释药率为91.89%±0.362%,金刚烷胺释药率为92%±0.362%。结论采用GAPI、AGREE和AES标准对所建立的分析方法进行了绿色度评价。然后将结果与文献中先前发表的方法进行比较。对于纳米颗粒中金刚烷胺和左旋多巴的同时测定,我们创新的UFLC-MS/MS技术提供了一种可靠且灵敏度极高的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A Novel and Green UFLC-MS/MS Method for Quantification of Amantadine and Levodopa in Polymeric Nanoparticles: Application to determine Drug loading (%DL), Drug entrapment (%DEE) and Drug release profile
Introduction
Levodopa, a dopamine precursor, widely prescribed drug in Parkinson’s disease management possesses a side effect of Levodopa-induced dyskinesia (LID). Amantadine hydrochloride, an NMDA receptor antagonist is co-encapsulated along with levodopa and formulates as polymeric nanoparticles (NPs) so as to overcome the side effects and act synergistically to enhance therapeutic outcome.
Methodology
In current work, we developed a novel, green Ultra-Fast Liquid Chromatography-Tandem Mass Spectrometry (UFLC-MS/MS) technique for the simultaneous quantification of amantadine and levodopa in polymeric nanoparticles. A triple quadrupole analyser with a multiple reaction monitoring (MRM) scan mode and an atmospheric pressure chemical ionization (APCI) source. A Waters Symmetry C8 column (150 × 4.6 mm, 3.5 μm) maintained at 40 °C was used for the chromatographic separation. In order to ensure sensitive and specific analyte detection, the mobile phase consisted of 0.1 % formic acid in water and methanol (40:60) with a total run time of 5 min. Excellent linearity, recovery, accuracy, and sensitivity were validated by method validation. Greenness assessment was done by AGREE, GAPI and AES metrics.
Results
Proposed green UFLC-MS/MS method effectively quantifies Levodopa and Amantadine in polymeric nanoparticles, followed by accurate evaluation of %DL, %DEE, and drug release profiles. %DEE values were observed for Levodopa (89.6%) and Amantadine (90.16 %), with corresponding %DL values of 20.5% and 24.10%, indicating substantial drug loading capacity. The in vitro drug release profiles demonstrated sustained release behaviour, with 91.89% ± 0.362 of Levodopa and 92% ± 0.362 of Amantadine released over the study period.
Conclusion
Using GAPI, AGREE, and AES criteria, the created analytical method's greenness was carefully assessed. The outcomes were then compared to previously published methods in the literature. For the simultaneous measurement of amantadine and levodopa in nanoparticles, our innovative UFLC-MS/MS technology offered a dependable and extremely sensitive method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


