具有OER和HER界面效应的NiFe2O4/NiO/g-C3N4电催化剂的合理构建
IF 5.1
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
摘要
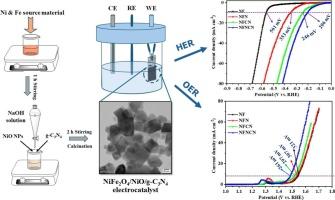
近年来,三元组合电催化剂因其独特的理化特性而引起了人们的广泛关注。本研究通过水热途径成功构建了NiFe2O4/NiO/g-C3N4三元电催化剂,实现了电催化水裂解。结构分析(XRD)表明该三元电催化剂具有良好的晶体质量。XPS分析证实了三元电催化剂中所有元素的存在。FESEM分析显示了所有电催化剂的表面形貌。通过TEM分析证实了NiFe2O4、NiO和g-C3N4的耦合形成。电化学水分解分析表明,三元纳米复合材料在HER和OER中均表现出增强的性能。具体而言,优化后的NiFe2O4/NiO/g-C3N4电催化剂在10 mA/cm2下具有261 mV的低电位和83 mV/dec的小Tafel斜率值,具有良好的OER效果。对于HER,三元电催化剂在10 mA/cm2下的过电位为206 mV,塔菲尔斜率值较小,为73 mV/dec。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Rational construction of NiFe2O4/NiO/g-C3N4 electrocatalyst with interfacial effects for OER and HER
The electrocatalysts with ternary combinations have recently attracted remarkable attention owing to their physio-chemical characteristics. Here, we successfully constructed the ternary NiFe2O4/NiO/g-C3N4 electrocatalyst via a hydrothermal route to facilitate electrocatalytic H2O splitting. The structural (XRD) analysis of the ternary electrocatalyst reveals good crystalline quality. The XPS analysis confirmed the presence of all the elements in the ternary electrocatalyst. The FESEM analysis shows the surface morphology of all the electrocatalysts. The coupled formation of NiFe2O4, NiO, and g-C3N4 was confirmed by TEM analysis. Electrochemical water splitting analysis reveals that the ternary nanocomposites exhibit enhanced performance in both HER and OER. Specifically, the optimized NiFe2O4/NiO/g-C3N4 electrocatalyst shows promising OER outcomes with a low potential of 261 mV at 10 mA/cm2 and a small Tafel slope value of 83 mV/dec. For HER, the ternary electrocatalyst shows an overpotential of 206 mV at 10 mA/cm2 with a small Tafel slope value of 73 mV/dec.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


