可见光诱导级联磷酸化/环化以获得含磷重氮氨基咔唑
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-05-16
DOI:10.1021/acs.joc.5c0071310.1021/acs.joc.5c00713
引用次数: 0
摘要
在此,我们开发了一种新的自由基级联磷酸化/环化策略,以中等到较高的产量获得含磷重氮嘧啶融合咔唑,为探索生物活性分子提供了更多的机会。该方法具有无金属光化学串联、条件温和、底物范围广、放大能力强等特点。机理实验表明,该光催化磷酸化/环化反应是通过EnT/SET/SET/自由基加成/环化/SET/去质子化等一系列过程实现的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
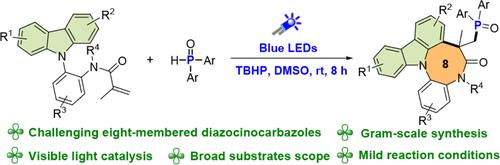
Visible Light-Induced Cascade Phosphorylation/Cyclization to Access Phosphorus-Containing Diazocinocarbazoles
Herein, we developed a novel radical cascade phosphorylation/cyclization strategy to access phosphorus-containing diazocine-fused carbazoles in moderate to good yields, offering expanded opportunities for the exploration of biologically active molecules. This method features metal-free photochemical tandem, mild conditions, broad substrate scopes, and scale-up ability. Mechanistic experiments implied that this photocatalyzed phosphorylation/cyclization reaction was realized by a sequence of EnT/SET/SET/radical addition/cyclization/SET/deprotonation processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


