跨组织多细胞协调及其在癌症中的重新布线
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
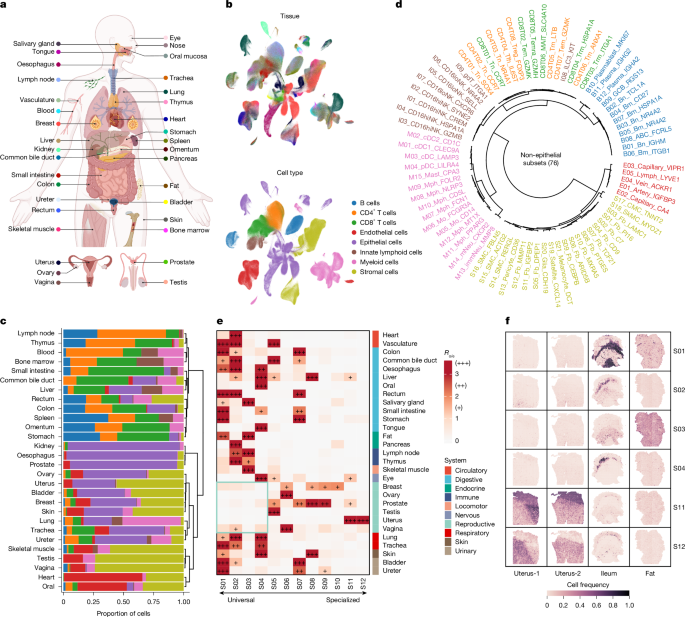
组织稳态和疾病进展背后的多细胞协调是最基本的兴趣1,2,3,4,5。然而,不同的细胞类型是如何组织在组织龛内的凝聚力功能仍然很大程度上是未知的。在这里,我们系统地表征了健康组织中的跨组织协调细胞模块,揭示了它们的时空动态和表型关联,并研究了它们在癌症中的重新布线。我们首先从35个人体组织中编制了一个全面的单细胞转录组图谱,揭示了细胞组成的组织间变异性。通过利用细胞丰度的协方差,我们确定了12个具有不同细胞组成、组织患病率和空间组织的细胞模块,并利用原位空间和体内扰动数据证明了细胞模块内协调的细胞间通信。其中,脾脏中的两个免疫细胞模块随着年龄的增长呈现出截然不同的时间动态。对乳腺多细胞变化的分析揭示了与成纤维细胞动力学相关的绝经期轨迹。此外,对不同癌症类型的研究发现,在肿瘤进展过程中,两种多细胞生态系统同时发生了重新连接,包括组织特异性健康组织的丧失和趋同癌性生态系统的出现。这些发现揭示了健康和癌症中多细胞生态系统的基本组织原则,为进一步研究不同背景下组织水平的功能协调奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Cross-tissue multicellular coordination and its rewiring in cancer
The multicellular coordination that underlies tissue homeostasis and disease progression is of fundamental interest1–5. However, how diverse cell types are organized within tissue niches for cohesive functioning remains largely unknown. Here we systematically characterized cross-tissue coordinated cellular modules in healthy tissues, uncovering their spatiotemporal dynamics and phenotypic associations, and examined their rewiring in cancer. We first compiled a comprehensive single-cell transcriptomic atlas from 35 human tissues, revealing substantial inter-tissue variability in cellular composition. By leveraging covariance in cellular abundance, we identified 12 cellular modules with distinct cellular compositions, tissue prevalences and spatial organizations, and demonstrated coordinated intercellular communication within cellular modules using in situ spatial and in vivo perturbation data. Among them, two immune cellular modules in the spleen showed contrasting chronological dynamics with ageing. Analysis of multicellular changes in the breast revealed a menopausal trajectory associated with fibroblast dynamics. Furthermore, interrogation across cancer types uncovered simultaneous rewiring of two types of multicellular ecosystem during tumour progression, including the loss of tissue-specific healthy organization and the emergence of a convergent cancerous ecosystem. These findings reveal fundamental organizing principles of multicellular ecosystems in health and cancer, laying a foundation for further investigations into tissue-level functional coordination across diverse contexts. Comprehensive single-cell transcriptomic analysis of 35 human tissues reveals 12 cross-tissue coordinated cellular modules that exhibit intramodule communication and dynamic changes with ageing, and whose organization is perturbed during cancer progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


