磺化和疏水聚糖对肝素酶驱动的肿瘤进展和转移的影响
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
肝素酶(HPSE)是唯一的哺乳动物内糖苷酶,它可以降解硫酸肝素(HS)蛋白聚糖,破坏细胞外基质(ECM),促进癌症的侵袭和转移。尽管HPSE过表达与肿瘤进展有关,但目前还没有临床批准的HPSE抑制剂。我们开发了基于氨基糖苷的HS模拟物,具有明确的磺化和疏水修饰,以靶向HPSE的亲脂口袋,这是一种不同于传统HS聚糖的新方法。计算模型表明,这些模拟物通过疏水和π -π堆叠相互作用参与HPSE,增强亲和力。最有效的化合物抑制hpse驱动的ECM降解,肿瘤细胞增殖和侵袭。在体内,主要候选药物显著降低了B16黑色素瘤和MPC-11骨髓瘤模型的转移负担,与SST0001 (TGI = 58.6%)相比,显示出肿瘤生长抑制(TGI = 83.1%),并与硼替佐米相匹配。重要的是,该化合物耐受性良好,没有明显的毒性。这些结果支持HPSE作为癌症靶点,并强调基于氨基糖苷的HS模拟物是转移性癌症的有希望的治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
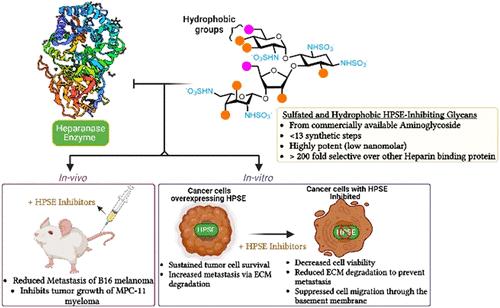
Design of Paromomycin and Neomycin as Sulfated and Hydrophobic Glycans to Target Heparanase-Driven Tumor Progression and Metastasis
Heparanase (HPSE) is the sole mammalian endoglycosidase that degrades heparan sulfate (HS) proteoglycans, disrupting the extracellular matrix (ECM) and promoting cancer invasion and metastasis. Although HPSE overexpression is linked to tumor progression, no clinically approved HPSE inhibitors exist. We developed aminoglycoside-based HS mimetics with defined sulfation and hydrophobic modifications to target HPSE’s lipophilic pockets, a novel approach distinct from traditional HS glycans. Computational modeling showed that these mimetics engage HPSE through hydrophobic and π–π stacking interactions, enhancing affinity. The most potent compounds inhibited HPSE-driven ECM degradation, tumor cell proliferation, and invasion. In vivo, the lead candidate significantly reduced metastatic burden in B16 melanoma and MPC-11 myeloma models, showing tumor growth inhibition (TGI = 83.1%) versus SST0001 (TGI = 58.6%) and matching bortezomib. Importantly, the compound was well-tolerated with no notable toxicity. These results support HPSE as a cancer target and highlight aminoglycoside-based HS mimetics as promising therapeutics for metastatic cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


