HAT化学辅助下的聚甲基丙烯酸酯主链引发解聚的原位改性
IF 5.2
Q1 POLYMER SCIENCE
引用次数: 0
摘要
在这项研究中,我们提出了一种新的策略来增强非功能化聚甲基丙烯酸甲酯(PMMA)的解聚,通过光诱导氢原子转移(HAT)化学使聚合物主链原位活化。通过筛选各种基于二硫化物的RAFT试剂,我们确定了市售的十二烷基磺酰硫代羰基二硫化物(DisRAFT-1)是最有效的,在四氯乙烷(TCE)中,在150°C和405 nm光照射下,在5小时内实现高达53%的单体回收率。系统研究了关键反应参数,包括DisRAFT-1负载、温度和聚合物浓度(10-200 mM),证明了该方法的效率和通用性。重要的是,我们强调光、升高的温度和氯化溶剂都是引发解聚的必要条件。此外,通过光开/关循环实现了对过程的时间控制,从而实现了按需解聚。这项工作为在温和和可调条件下利用原位主链活化非功能化聚合物的化学回收提供了一条有前途的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
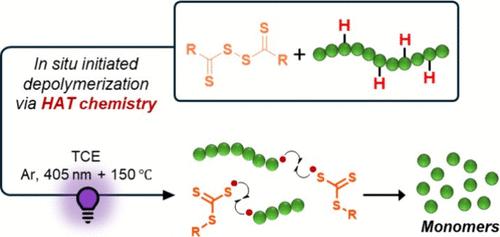
In Situ Modification Assisted by HAT Chemistry for the Main Chain Initiated Depolymerization of Polymethacrylates
In this study we present a novel strategy to enhance the depolymerization of nonfunctionalized poly(methyl methacrylate) (PMMA) by enabling in situ activation of the polymer backbone using photoinduced Hydrogen Atom Transfer (HAT) chemistry. By screening various disulfide-based RAFT agents, we identified the commercially available bis(dodecylsulfanylthiocarbonyl) disulfide (DisRAFT-1) as the most effective, achieving up to 53% monomer recovery within 5 h at 150 °C under 405 nm light irradiation in tetrachloroethane (TCE). A systematic investigation of key reaction parameters, including DisRAFT-1 loading, temperature, and polymer concentration (10–200 mM), demonstrated the efficiency and versatility of the approach. Importantly, we highlight that light, elevated temperature, and a chlorinated solvent are all essential to initiate depolymerization. Moreover, temporal control over the process was achieved via light ON/OFF cycles, enabling on-demand depolymerization. This work offers a promising route toward chemical recycling of nonfunctionalized polymers by leveraging in situ backbone activation under mild and tunable conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.40
自引率
3.40%
发文量
209
审稿时长
1 months
期刊介绍:
ACS Macro Letters publishes research in all areas of contemporary soft matter science in which macromolecules play a key role, including nanotechnology, self-assembly, supramolecular chemistry, biomaterials, energy generation and storage, and renewable/sustainable materials. Submissions to ACS Macro Letters should justify clearly the rapid disclosure of the key elements of the study. The scope of the journal includes high-impact research of broad interest in all areas of polymer science and engineering, including cross-disciplinary research that interfaces with polymer science.
With the launch of ACS Macro Letters, all Communications that were formerly published in Macromolecules and Biomacromolecules will be published as Letters in ACS Macro Letters.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


