富氧无定形碳微球通过氢键作用增强了FeC2O4阳极的储锂能力
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
草酸铁(α-FeC2O4)具有稳定的Li+扩散途径,具有良好的循环稳定性,但由于其电子导电性低,限制了其商业应用。为了克服这一问题,我们引入了热分解后带有部分氧官能团的有机β-环糊精(β-CD)化合物,制备了非晶碳微球(AMCs)复合材料。考虑到FeC2O4·2H2O中间层之间结晶水的稳定支撑力,提出了一种通过氢键相互作用将α-FeC2O4与AMCs (FCO/AMCs)复合的独特工艺。通过调整溶剂比,FCO/AMCs 1:3具有较高的氧空位浓度和AMCs建立的导电网络,在电流密度为1 A g-1时,循环1000次后的放电比容量为1183.78 mAh g-1,在5 A g-1下,循环750次后的容量保持率为72.8%。基于氧空位的催化作用和更快的平均Li+扩散速率,FCO/AMCs 1:3电极还具有更高的交换电流密度(j0 = 2.35 × 10-3 A cm-2)和表观电子转移速率常数(kapp = 1.02 cm s-1),具有更高的可逆性。此外,深入分析了锂离子在转化反应中的动力学参数倾向性行为差异,发现更多的氧空位可以促进Fe0的电离,释放更多的自旋极化电子,形成自旋极化电容。该研究为碳复合提供了一种新的策略,并对草酸盐基阳极材料的动力学过程有了更全面的了解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
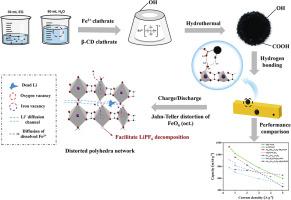

Enhancing Li-storage ability of FeC2O4 anode enabled by oxygen-vacancy-enriched amorphous carbon microspheres compositing via hydrogen bonding interactions
Iron oxalate (α-FeC2O4), with a stable Li+ diffusion pathway, exhibits excellent cycling stability but is limited in commercial applications due to its low electronic conductivity. To overcome this issure, we introduced an organic β-cyclodextrin (β-CD) compound with partial oxygen functional groups after thermal decomposition, to prepare an amorphous carbon microspheres (AMCs) composite. Considering the stable sustaining force supported by crystal water between FeC2O4·2H2O interlayer, a unique composite technology with α-FeC2O4 and AMCs through the interactions of hydrogen bonds (FCO/AMCs) was proposed. By adjusting the solvent ratio, FCO/AMCs 1:3 with high concentration of oxygen vacancies and conductive network established by AMCs, exhibits excellent discharge specific capacity of 1183.78 mAh g-1 at current density of 1 A g-1 after 1000 cycles and stable cycling performance with the capacity retention rate of 72.8 % even over 750 cycles at 5 A g-1. Based on the catalytic effect of oxygen vacancies and faster average Li+ diffusion rate, FCO/AMCs 1:3 electrode also shows higher exchange current density (j0 = 2.35 × 10–3 A cm-2) and apparent electron transfer rate constant (kapp = 1.02 cm s-1), leading higher reversibility. Additionally, an in-depth analysis of kinetic parameters Li-ion tendentious behavior differences in transformation reactions, reveals that more oxygen vacancies can promote the ionization of Fe0, releasing more spin-polarized electrons to form spin-polarized capacitance. This research provides a novel strategy on carbon compositing and offers more comprehensive understanding of the kinetic processes involved in the oxalate-based anode material.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


