在氧化条件下,无金属HFIP促进二苯乙烯作为掩蔽亲电试剂合成苯甲酸衍生物
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
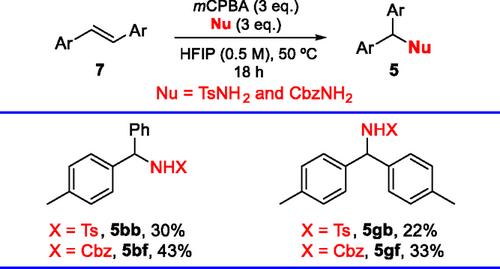
在亲核取代反应中,利用二苯乙烯作为亲电反应伙伴,通过非极性反应性合成了苯并羟基衍生物。该转化需要用mCPBA进行氧化处理,并以1,1,1,3,3,3 -六氟异丙醇(HFIP)作为必需的溶剂和反应促进剂,需要经过多个连续步骤进行。在此条件下,将二苯乙烯与多种氮基、氧基、硫基和碳基亲核试剂偶联,合成了不同的苯并羟基衍生物。本文章由计算机程序翻译,如有差异,请以英文原文为准。


Metal-Free HFIP-Promoted Synthesis of Benzhydryl Derivatives from Stilbenes as Masked Electrophiles under Oxidative Conditions
Benzhydryl derivatives are synthesized by a new strategy involving the use of stilbenes as an electrophilic reaction partner through umpolung reactivity in a nucleophilic substitution reaction. The transformation, which requires oxidative treatment with meta-chloroperoxybenzoic acid and the presence of 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) as an essential solvent and reaction promoter, occurs through multiple consecutive steps. Under these conditions, different benzhydryl derivatives are synthesized by coupling stilbenes with a variety of nitrogenated, oxygenated, and sulfur- and carbon-based nucleophiles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


