人自噬启动ULK1C:PI3KC3-C1超复合体的结构和激活
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
unc -51样激酶蛋白激酶复合体(ULK1C)是哺乳动物巨噬起始的最上游和最核心的参与者。在这里,我们以氨基酸水平的分辨率确定了人类ULK1C核心的低温电镜结构。我们还确定了ULK1C核心复合物与另一个自噬核心复合物的中等分辨率结构,III类磷脂酰肌醇3-OH激酶复合物I (PI3KC3-C1)。我们发现这两个复合物通过ULK1C的FIP200支架亚基与PI3KC3-C1的VPS15、ATG14和BECN1亚基之间的广泛接触而聚集在一起。在PI3KC3-C1存在下,ULK1C的FIP200:ATG13:ULK1核心经历了从2:1:1到2:2:2的化学计量重排。这表明自噬起始的结构机制是通过ULK1C:PI3KC3-C1超复合物的形成和ULK1在FIP200支架上的二聚化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
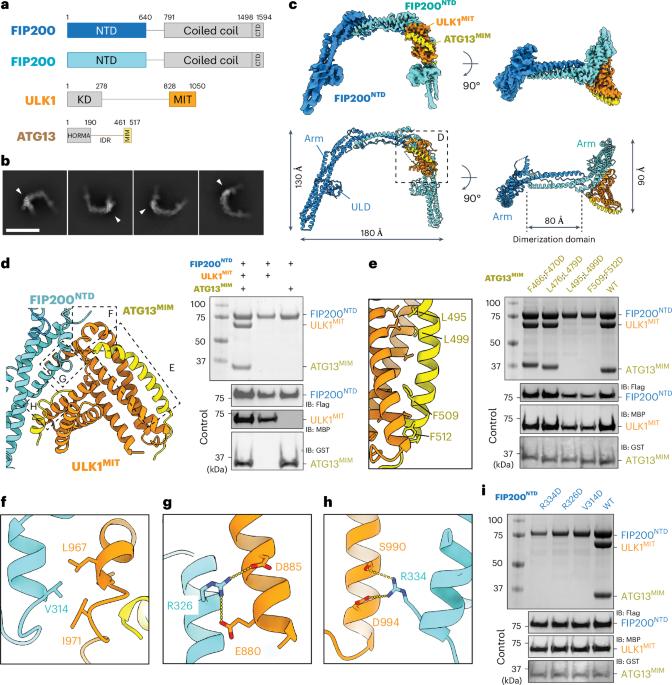

Structure and activation of the human autophagy-initiating ULK1C:PI3KC3-C1 supercomplex
The Unc-51-like kinase protein kinase complex (ULK1C) is the most upstream and central player in the initiation of macroautophagy in mammals. Here, we determined the cryo-electron microscopy structure of the human ULK1C core at amino-acid-level resolution. We also determined a moderate-resolution structure of the ULK1C core in complex with another autophagy core complex, the class III phosphatidylinositol 3-OH kinase complex I (PI3KC3-C1). We show that the two complexes coassemble through extensive contacts between the FIP200 scaffold subunit of ULK1C and the VPS15, ATG14 and BECN1 subunits of PI3KC3-C1. The FIP200:ATG13:ULK1 core of ULK1C undergoes a rearrangement from 2:1:1 to 2:2:2 stoichiometry in the presence of PI3KC3-C1. This suggests a structural mechanism for the initiation of autophagy through formation of a ULK1C:PI3KC3-C1 supercomplex and dimerization of ULK1 on the FIP200 scaffold. Autophagy is initiated by the Unc-51-like kinase protein kinase complex (ULK1C) and class III phosphatidylinositol 3-OH kinase complex I (PI3KC3-C1). Here, the authors reveal the structure of the 2:1:1 core of ULK1C and its complex with PI3KC3-C1. ULK1C transitions to a 2:2:2 complex in the presence of PI3KC3-C1, suggesting a mechanism for autophagy induction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


