CD4+ T细胞n -糖基化随graves病的发展而变化,对甲巯咪唑治疗敏感。
IF 2.2
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. General subjects
Pub Date : 2025-05-25
DOI:10.1016/j.bbagen.2025.130824
引用次数: 0
摘要
格雷夫斯病(GD)是最常见的自身免疫性疾病之一。辅助性T (Th)细胞,其表面受体富含聚糖,参与GD的病理机制。n -糖基化在自身免疫过程中发生改变,可通过药物治疗进行调节。我们假设GD伴有Th糖基化的变化,这些细胞的糖基化对甲巯咪唑治疗敏感。研究组为经甲巯咪唑治疗恢复甲亢前(GD/T)和后(GD/T) Graves病患者。在对照组中,招募健康的供体。这些细胞从pbmc中分离出来,并分为CD4+CD25-细胞亚群和表达CD25晚期激活标志物(CD4+CD25+)的细胞亚群。采用MALDI-Tof质谱法分析n链聚糖,RT-qPCR法检测糖基转移酶的表达。CD4+细胞亚群的n -糖基化谱在GD中复合物与寡甘露糖n -聚糖的比例不同。在GD的CD4+CD25-细胞中,n -甘聚糖复合物部分被寡甘露糖形式所取代,其结构因无半乳糖化而缩短。CD4+CD25+细胞中n -聚糖的重排方向相反,即在GD中比例向复杂结构转移。n -聚糖谱的变化部分反映在MGAT5和FUT8的表达上。甲巯咪唑在一定程度上使糖基转移酶水平正常化,并影响n链聚糖谱。我们的研究首次揭示了GD发展和甲巯咪唑治疗中CD4+ T细胞n -糖基化的变化。需要进一步的研究来确定甲状腺自身免疫中糖基化变化的功能方面。本文章由计算机程序翻译,如有差异,请以英文原文为准。
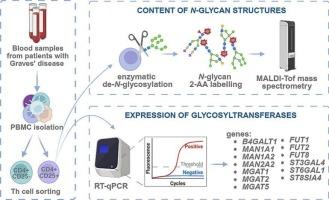
N-glycosylation of CD4+ T cell changes with the development in Graves' disease and is sensitive to methimazole treatment
Graves' disease (GD) is one of the most common autoimmune disorders. Helper T (Th) cells, whose surface receptors are rich in glycans, are involved in the GD pathomechanism. N-glycosylation is altered during autoimmunity and can be modulated by pharmacotherapy. We hypothesized that changes in Th glycosylation accompany GD, and the glycome of these cells is sensitive to methimazole therapy. The study group consisted of patients with Graves' disease before (GD) and after (GD/T) restoring euthyroidism as a result of methimazole therapy. In the control group, healthy donors were recruited. Th cells were isolated from PBMCs and sorted into a subpopulation of CD4+CD25− cells and those expressing the CD25 late activation marker (CD4+CD25+). MALDI-Tof MS was used for analysis of N-linked glycans, and the expression of glycosyltransferases was determined by RT-qPCR. The N-glycosylation profile of CD4+ cell subpopulations differed in the ratio of the complex-to-oligomannose N-glycans in GD. Complex N-glycans are partially replaced by oligomannose forms, and their structure is shortened by agalactosylation in CD4+CD25− cells from GD. The rearrangement of N-glycans in CD4+CD25+ cells has the opposite direction, namely the ratio is shifted towards complex structures in GD. The changes in the N-glycan profile were reflected partly in MGAT5 and FUT8 expression. Methimazole to some extent normalized the glycosyltransferase levels and affected the N-linked glycans profile. Our study shows N-glycosylation changes in CD4+ T cells in GD development and methimazole therapy for the first time. Further studies are needed to determine the functional aspect of the identified glycosylation changes in thyroid autoimmunity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. General subjects
生物-生化与分子生物学
CiteScore
6.40
自引率
0.00%
发文量
139
审稿时长
30 days
期刊介绍:
BBA General Subjects accepts for submission either original, hypothesis-driven studies or reviews covering subjects in biochemistry and biophysics that are considered to have general interest for a wide audience. Manuscripts with interdisciplinary approaches are especially encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


