椰氨酸盐阴离子行为对活性炭吸附烟头铅(II)离子的影响
Q1 Environmental Science
Case Studies in Chemical and Environmental Engineering
Pub Date : 2025-02-06
DOI:10.1016/j.cscee.2025.101140
引用次数: 0
摘要
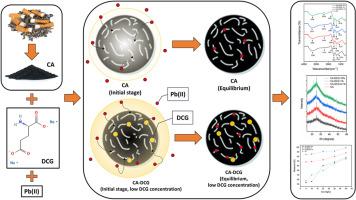
烟头是世界上最丰富的废物之一,由于其不可生物降解的特性和剧毒,它是危险的。烟头可以转化为活性炭(CA),作为吸附剂。然而,合成的CA缺乏有利于与吸附物相互作用的官能团,如重金属。研究了椰子酰谷氨酸二钠(DCG)改性对钙吸附铅(II)离子的影响。以烟头废料为原料,经过闪蒸炭化和活化等关键工艺合成CA。本研究使用的表面活性剂是DCG,因为它具有自然和阴离子的性质,不同浓度(0.1,1和10% (v/v))。红外光谱分析表明,CA和CA- dcg的结构主要是由羰基和羟基等氧官能团官能化的芳香环组成。通过红外和元素分析数据证实,添加DCG可以增加CA中羰基的数量。所得的CA-DCGs具有非晶性质和微孔结构。DCG的存在也会影响CA-DCG的结晶度,随着DCG浓度的增加,CA-DCG的非晶态含量增加。结果表明,添加DCG后,CA的平衡去除率由25%提高到99%,其中0.1%的CA-DCG的最大吸附量为9.89 mg/g。由于DCG的阴离子行为,DCG的存在显著增强了CA对铅离子的亲和力。基于这些结果,CA-DCG具有作为铅(II)离子吸附剂的潜力。它还可以减少环境中现有的烟头废物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Significant role of anionic behavior of cocoyl glutamate on lead(II) ion adsorption towards activated carbon adsorbent from cigarette butts
Cigarette butts are one of the most abundant waste in the world, being dangerous due to non-biodegradable behavior and highly toxic. Cigarette butts can be converted to activated carbon (CA) which can be used as an adsorbent. However, the synthesized CA lacks functional groups facilitating the interaction with adsorbate, like heavy metal. This paper investigates the effect of Disodium Cocoyl Glutamate (DCG) modification on CA towards lead(II) ion adsorptions. CA was synthesized from cigarette butt waste through several vital processes, like flash carbonization and activation. The surfactant used in this study was DCG due to its natural and anionic behavior, with varied concentrations (0.1, 1, and 10 % (v/v)). Infrared spectroscopy revealed that the structure of both CA and CA-DCG mainly consists of aromatic rings functionalized by several oxygen functional groups, such as carbonyl and hydroxyl groups. Adding DCG increases the number of carbonyl groups in the CA confirmed by infrared and elemental analysis data. The obtained CA-DCGs have an amorphous property and a slightly porous structure. The presence of DCG also affects CA-DCG crystallinity, which increases the amorphous content as the concentration of DCG increases. The adsorption results show that the equilibrium removal efficiency significantly increased from 25 % to 99 % as DCG was introduced to the CA. CA-DCG 0.1 % demonstrated the highest maximum adsorption capacity of 9.89 mg/g. The presence of DCG markedly enhances the affinity of CA towards lead(II) ions due to its anionic behavior. Based on these results, CA-DCG is potentially applied as a lead(II) ion adsorbent. It also could reduce the existing cigarette butt waste in the environment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Case Studies in Chemical and Environmental Engineering
Engineering-Engineering (miscellaneous)
CiteScore
9.20
自引率
0.00%
发文量
103
审稿时长
40 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


