Ti(II)的叠氮化物加合物与三烷基叠氮化物的合成及其异构体
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
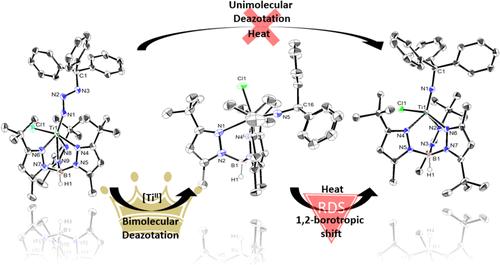
本文报道了Ti(II)前驱体[(Tp3-tBu,5-Me)TiCl] (1,tp3 - tbu,5-Me =羟基(三(3-甲基-5-叔丁基吡唑)硼酸盐)通过三叠氮基双分子脱氮法制备了体积较大的钛(IV)亚胺。叠氮化物基团上的立体阻碍三烷基取代基阻碍了单分子脱氮途径,使得Ti的稀有有机叠氮化物加合物,即重氮酰亚胺配合物[(Tp3-tBu,5-Me)Ti(N3CPh3)Cl](2)的分离和充分表征成为可能。我们展示了自由Ti(II)是促进脱氮的必要条件,从而允许2的双分子转化为空间拥挤的三苯基氨基配合物[(Tp3-tBu,5-Me)Ti(NCPh3)Cl](3)。配合物3是由2脱氮形成的动力学产物,温和的热裂解导致其异构体[{HB(pyztBu,Me)2(pyzMe,tBu)}Ti{NCPh3}Cl] (4), {HB(pyztBu,Me)2(pyzMe,tBu)} =羟基(双(3-甲基-5-叔丁基吡唑基)(5-甲基-3-叔丁基吡唑基)硼酸盐)通过1,2-亲硼位移形成。这项研究表明,2到3的单分子脱氮是明显不利的,这是由于三甲基取代基的空间体积。通过堵塞亚胺片段,我们还从机制上展示了3的转换是如何发生的,以减轻CPh3基团形成4的堵塞。通过VT-1H核磁共振波谱测量,该过程涉及一个限速的1,2-硼向位移,激活参数Ea = 30(1) kcal/mol, ΔH‡= 30(1)kcal/mol, ΔS‡= 15(1)cal/mol·K。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An Azide Adduct of Ti(II) with a Sterically Encumbering Trityl Azide and Independent Synthesis of a Bulky Ti(IV) Imido and Its Isomer
We report here the synthesis and characterization of a bulky titanium(IV) imido via the bimolecular deazotation of trityl azide by the Ti(II) precursor [(Tp3-tBu,5-Me)TiCl] (1, Tp3-tBu,5-Me = hydrido(tris(3-methyl-5-tert-butylpyrazolyl)borate)). The sterically encumbering trityl substituent on an azide group discourages the unimolecular deazotation pathway and allows the isolation and full characterization of a rare organic azide adduct of Ti, namely, the diazenylimide complex [(Tp3-tBu,5-Me)Ti(N3CPh3)Cl] (2). We show how free Ti(II) is a necessity to promote deazotation, thus allowing for a bimolecular conversion of 2 to a sterically crowded triphenylimido complex, [(Tp3-tBu,5-Me)Ti(NCPh3)Cl] (3). Complex 3 is the kinetic species formed from deazotation of 2, and mild thermolysis results in the formation of its isomer [{HB(pyztBu,Me)2(pyzMe,tBu)}Ti{NCPh3}Cl] (4), {HB(pyztBu,Me)2(pyzMe,tBu)} = hydrido(bis(3-methyl-5-tert-butylpyrazolyl)(5-methyl-3-tert-butylpyrazolyl)borate) via a 1,2-borotropic shift. This study demonstrates that unimolecular deazotation of 2 to 3 is significantly disfavored, and attributed to the steric bulk of the trityl substituent. By congesting the imido fragment, we also show mechanistically how conversion of 3 takes place to alleviate congestion by the CPh3 group to form 4. This process involves a rate-limiting 1,2-borotropic shift with activation parameters Ea = 30(1) kcal/mol, ΔH‡ = 30(1) kcal/mol, and ΔS‡ = 15(1) cal/mol·K as measured by VT-1H NMR spectroscopy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


