级联催化和光热双增强化学动力学治疗的含多金属氧酸纳米复合水凝胶
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
化学动力疗法(CDT)已经成为活性氧(ROS)介导的癌症治疗领域的一个变革范例。然而,肿瘤中缺乏内源性过氧化氢(H2O2),传统Fenton催化剂的催化效率低,限制了CDT的治疗效果。本文设计了一种基于透明质酸-多巴胺(HA-DOPA)基质的可注射纳米复合水凝胶(HA-DOPA/W-POM/1-S-S-PEG@GOx),用于递送钨基多金属氧酸盐(W-POM)和肽纳米束(1-S-S-PEG@GOx),以实现级联催化和光热双重增强CDT。在肿瘤细胞摄取后,1-S-S-PEG@GOx特异性响应内源性谷胱甘肽并分解释放葡萄糖氧化酶(GOx),该酶催化葡萄糖氧化产生H2O2。一方面,W-POM作为过氧化物酶类纳米酶,在GOx的作用下将H2O2转化为羟基自由基(·OH),通过级联催化反应(即葡萄糖将H2O2转化为·OH)增强CDT的功效。另一方面,W-POM作为光热治疗剂,在近红外激光照射下产生微热,实现光热增强CDT。这种级联催化和光热双重增强的CDT触发细胞内ROS风暴,导致肿瘤细胞凋亡和铁凋亡。重要的是,原位给药HA-DOPA/W-POM/1-S-S-PEG@GOx与激光照射显示出增强的抗肿瘤效果和令人满意的体内生物相容性,这在功能纳米药物靶向肿瘤治疗方面具有很大的发展潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
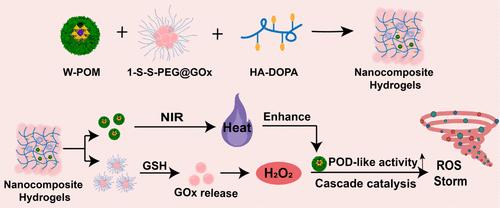
Polyoxometalate-Containing Nanocomposite Hydrogels for Cascade-Catalytic and Photothermal Dually Enhanced Chemodynamic Therapy
Chemodynamic therapy (CDT) has emerged as a transformative paradigm in the realm of reactive oxygen species (ROS)-mediated cancer therapies. However, the lack of endogenous hydrogen peroxide (H2O2) in tumors and the low catalytic efficiency of traditional Fenton catalysts limit the therapeutic effect of CDT. Herein, an injectable nanocomposite hydrogel (HA-DOPA/W-POM/1-S-S-PEG@GOx) based on the hyaluronic acid-dopamine (HA-DOPA) matrix is designed to deliver tungsten-based polyoxometalates (W-POM) and peptide nanomicelles (1-S-S-PEG@GOx) for achieving cascade-catalytic and photothermal dually enhanced CDT. Upon tumor cell uptake, 1-S-S-PEG@GOx specifically responds to endogenous glutathione and disassembles to release glucose oxidase (GOx), which catalyzes the oxidation of glucose to produce H2O2. On the one hand, W-POM functions as peroxidase-like nanozymes to convert H2O2 into a hydroxyl radical (·OH) under the aid of GOx, enhancing the efficacy of CDT through cascade-catalytic reactions (i.e., glucose to H2O2 to ·OH). On the other hand, W-POM acts as a photothermal therapy agent, generating mild heat under near-infrared laser irradiation to achieve photothermal-enhanced CDT. This cascade-catalytic and photothermal dually enhanced CDT triggers an intracellular ROS storm, leading to apoptosis and ferroptosis of tumor cells. Importantly, in situ administration of HA-DOPA/W-POM/1-S-S-PEG@GOx alongside laser irradiation showcases enhanced antitumor efficacy and satisfactory biocompatibility in vivo, which holds great potential for the development of functional nanomedicine toward targeted tumor therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


