带极性拉链的自组装肽纳米带证明了酶模拟活性位点的裂缝
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
由于天然酶的固有局限性,仿生酶受到了极大的关注,其中由肽自组装产生的仿生酶由于其在组成和层次结构上与天然酶相似,以及其结构稳健性和可设计性而受到特别关注。尽管在这一领域取得了相当大的进展,但通过肽自组装构建活性位点裂缝仍然是一个重大挑战。在这里,我们报道了在肽β-片之间设计极性拉链来模拟天然酶的催化微环境。极性拉链作为一种由侧链-侧链氢键稳定的超二级结构基序,不仅促进β-片层合形成宽纳米带,而且在纳米带表面形成裂缝。在设计的三种肽类似物(I3GH、I3GHK和I3HGK)中,组氨酸(His或H)极性拉链仅在I3HGK的自组装中形成,从而形成较宽的I3HGK纳米带和较薄的I3GH和I3GHK纳米原纤维。与I3GHK和I3GH纳米原纤维相比,I3HGK纳米带在水解对硝基苯乙酸(pNPA)方面表现出显著提高的催化效率,这是由于埋在裂缝中的极性反应性His残基和疏水性Ile(I)残基的协同相互作用。通过在裂口内用其他不带电的极性残基取代His,可以调整肽纳米带的催化能力,其中I3CGK纳米带在pNPA水解中表现出最高的催化效率,这是因为半胱氨酸(Cys或C)侧链具有强大的亲核性。这项工作为通过短肽的合理设计和自组装来模拟天然酶的催化裂孔提供了一个新的概念框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
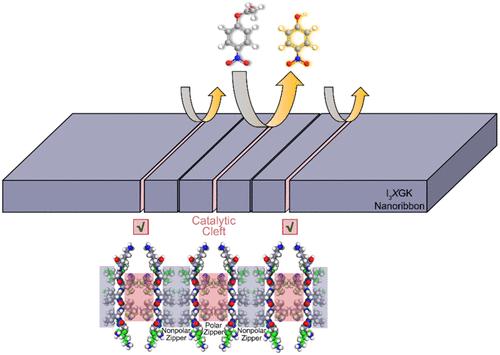
Enzyme-Mimicking Active Site Clefts Demonstrated by Self-Assembled Peptide Nanoribbons with Polar Zippers
Due to the inherent limitations of natural enzymes, biomimetic enzymes have received tremendous attention, among which those arising from peptide self-assembly are of particular interest due to their resemblance to natural enzymes in composition and hierarchical structures, as well as their structural robustness and designability. Despite considerable advances achieved in this area, it remains a major challenge to construct active site clefts through peptide self-assembly. Here, we report the design of polar zippers between peptide β-sheets to mimic the catalytic microenvironment of natural enzymes. As a supersecondary structural motif stabilized by the side chain–side chain hydrogen bonding, polar zippers not only promote significant β-sheet lamination to form wide nanoribbons but also constitute clefts on the nanoribbons’ surface. Among the three designed peptide analogues (I3GH, I3GHK, and I3HGK), histidine (His or H) polar zippers between β-sheets form only in the self-assembly of I3HGK, thus leading to the formation of wide I3HGK nanoribbons and thin I3GH and I3GHK nanofibrils. Compared to the I3GHK and I3GH nanofibrils, the I3HGK nanoribbons exhibit substantially increased catalytic efficiency in the hydrolysis of p-nitrophenyl acetate (pNPA) due to the synergistic interplay of polar reactive His residues and hydrophobic Ile(I) residues buried within the clefts. By substituting other uncharged polar residues for His within the clefts, the catalytic ability of the peptide nanoribbons can be tuned, with the I3CGK ones exhibiting the highest catalytic efficiency in the pNPA hydrolysis, owing to the potent nucleophilicity of the cysteine (Cys or C) side chain. This work offers a new conceptual framework for mimicking the catalytic cleft of natural enzymes through the rational design and self-assembly of short peptides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


