寻找金属烯醇化物的自由基转变。乙酸铜催化n-酰基-1,3-恶唑烷-2-酮与TEMPO的直接反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文描述了多种n-酰基-1,3-恶唑烷-2- 1与TEMPO在乙酸铜(II)和4,7-二甲基-1,10-菲罗啉催化下不需要任何碱的直接反应。这些反应提供了相应的氨基化衍生物,具有高的化学选择性和完全的区域选择性,在温和的条件下获得了优异的收率。进一步处理所得到的亚酰亚胺,可以得到各种形式上受保护的羟基化合物,这些羟基化合物可被视为有价值的合成中间体。异丙肾上腺素的高效正式合成突出了这种方法的潜力,并为金属烯醇酯催化化学的进一步发展奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
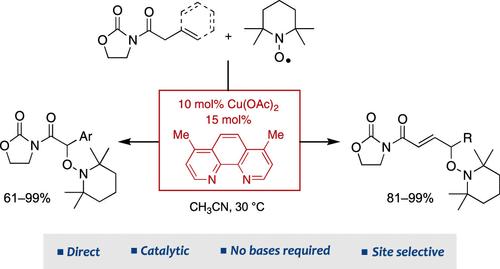
In Search of Radical Transformations from Metal Enolates. Direct Reactions of N-Acyl-1,3-oxazolidin-2-ones with TEMPO Catalyzed by Copper(II) Acetate
The direct reactions of a diverse range of N-acyl-1,3-oxazolidin-2-ones with TEMPO, catalyzed by copper(II) acetate and 4,7-dimethyl-1,10-phenanthroline without the need for any base, are herein described. These reactions afford the corresponding aminoxylated derivatives with high chemoselectivity and complete regioselectivity, achieving excellent yields under mild conditions. Further treatment of the resulting imides enables access to a variety of formally protected hydroxy compounds, which can be regarded as valuable synthetic intermediates. The efficient formal synthesis of isoproterenol highlights the potential of this methodology and sets the stage for further advancements in the catalytic chemistry of metal enolates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


