光氧化还原催化溴三氟丙酮与富电子(杂)芳烃的α-芳基化反应
IF 2
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在温和条件下建立了光氧化还原催化溴三氟丙酮和(杂)芳烃自由基加成羰基化合物的反应。所提出的方法能够合成有价值的α-芳基三氟甲基酮化合物,包括生物相关的芳烃衍生物,具有良好的产率和区域选择性。该方法使用Zn(OAc)2作为添加剂消除了竞争性溴化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
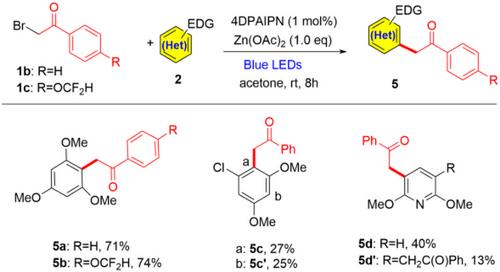
Photoredox-Catalyzed α-Arylation of Bromotrifluoroacetone with Electron-Rich (Hetero)Arenes
Photoredox-catalyzed radical addition of bromotrifluoroacetone and (hetero)arenes to carbonyl compounds has been established under mild conditions. The proposed method enables the synthesis of valuable α-aryl trifluoromethyl ketone compounds, including biologically relevant arene derivatives, with good yields and regioselectivities. This method eliminates competitive bromination using Zn(OAc)2 as an additive.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ChemistrySelect
Chemistry-General Chemistry
CiteScore
3.30
自引率
4.80%
发文量
1809
审稿时长
1.6 months
期刊介绍:
ChemistrySelect is the latest journal from ChemPubSoc Europe and Wiley-VCH. It offers researchers a quality society-owned journal in which to publish their work in all areas of chemistry. Manuscripts are evaluated by active researchers to ensure they add meaningfully to the scientific literature, and those accepted are processed quickly to ensure rapid online publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


