核壳ZnO@ZIF-8-derived氮掺杂碳纳米管用于甲基橙的有效去除
IF 8.7
Q1 Environmental Science
引用次数: 0
摘要
将沸石咪唑酸骨架-8 (ZIF-8)包覆在ZnO纳米棒上,通过热解、酸蚀法制备氮掺杂碳纳米管(NC)。形态学研究表明,NC为直径为194 nm的多孔管状结构。ZnO纳米棒上的ZIF-8模板在核心ZnO周围形成薄的壳状层。x射线衍射(XRD)分析显示NC的非晶结构,FTIR/XPS光谱证实NC中存在氮掺杂、ZnO@ZIF-8中Zn-N相互作用以及N-C环境。ZnO@ZIF-8、ZnO@NC和NC的BET表面积分别为724.81±12.77、638.92±1.29和787.11±4.26 m2 g−1。ZnO@NC和NC在10和20 mg L−1浓度下成功去除MO,平衡吸附量分别为50.23和100.50 mg g−1。ZnO@NC和NC的最佳条件为pH = 6,接触时间为60 min,吸附剂用量为0.2 g L−1。Langmuir等温线的高相关系数R2(0.9983)表明,纳米活性炭的吸附量最大,qmax = 126.58 mg g−1。NC服从拟二级动力学,具有较高的R2(0.999),证实了NC表面与mo之间的化学相互作用。ZnO@NC表现为化学吸附,而NC则表现为物理和化学吸附,通过等温线(D-R)和动力学(Elovich)模型进行了鉴定。结果表明,NC具有较大的BET表面积、管状结构、非均相氮掺杂碳和丰富的介孔通道,有利于有效吸附MO。本文章由计算机程序翻译,如有差异,请以英文原文为准。
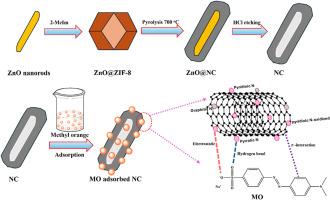
Core–shell ZnO@ZIF-8-derived nitrogen-doped carbon nanotubes for the effective removal of methyl orange
The nitrogen-doped carbon nanotubes (NC) are synthesized via pyrolysis of zeolitic imidazolate framework-8 (ZIF-8) coated on ZnO nanorods, followed by acid etching. Morphological studies reveal that the NC shows a porous, tube-like structure with a diameter of 194 nm. The ZIF-8 template on the ZnO nanorods forms thin, shell-like layers surrounding the core ZnO. X-ray diffraction (XRD) analysis reveals the amorphous structure of NC, while FTIR/XPS spectra affirm nitrogen doping, Zn-N interaction in ZnO@ZIF-8, and the N-C environment in NC. The BET surface areas for ZnO@ZIF-8, ZnO@NC, and NC are 724.81 ± 12.77, 638.92 ± 1.29, and 787.11 ± 4.26 m2 g−1, respectively. ZnO@NC and NC successfully removed MO at concentrations of 10 and 20 mg L−1, achieving equilibrium adsorption capacities of 50.23 and 100.50 mg g−1, respectively. The optimum conditions for ZnO@NC and NC were a pH of 6, a contact time of 60 min, and an adsorbent dosage of 0.2 g L−1, respectively. The high correlation coefficient R2 (0.9983) from the Langmuir isotherm indicated monolayer adsorption on NC with the maximum adsorption capacity (qmax = 126.58 mg g−1). NC followed pseudo-second-order kinetics with a high R2 (0.999), substantiating the chemical interaction between the NC surface and MO. ZnO@NC showed chemical adsorption, while NC underwent physical and chemical adsorption, as identified through the isotherm (D-R) and kinetic (Elovich) models. The results show that NC proves a large BET surface area, a tubular structure, heterogeneous nitrogen-doped carbon, and abundant mesoporous channels that beneficially facilitate effective MO adsorption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Cycle
Engineering-Engineering (miscellaneous)
CiteScore
9.20
自引率
0.00%
发文量
20
审稿时长
45 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


