血小板膜包被的载药纳米颗粒用于三阴性乳腺癌的双模成像和光动力治疗
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
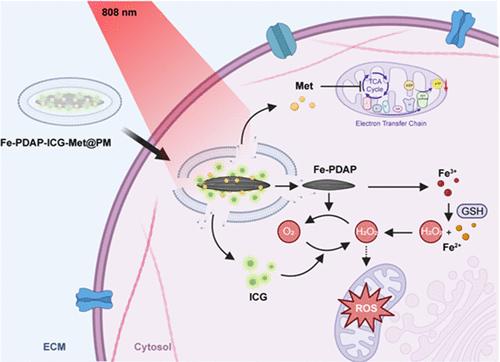
光动力疗法(PDT)已成为一种有前途的癌症治疗策略;然而,其疗效受到肿瘤微环境缺氧的影响。在这项研究中,我们设计了一个仿生纳米系统来增强氧依赖性PDT。该体系由铁掺杂的具有过氧化氢酶样活性的聚二氨基吡啶(Fe-PDAP)纳米酶组成,并用二甲双胍(Met)和吲哚菁绿(ICG)包裹。纳米颗粒的表面进一步被血小板膜(PM)包裹,从而通过分子识别靶向递送到肿瘤部位。体外和体内研究表明,Fe-PDAP纳米酶可以催化过氧化氢(H2O2)生成O2,同时消耗谷胱甘肽(GSH),从而增加活性氧(ROS)的产生,增强PDT的功效。Met作为线粒体呼吸抑制剂,破坏电子传递链的复合物I,从而降低ATP水平,抑制肿瘤部位的氧气消耗,并放大PDT效应。此外,仿生纳米颗粒(Fe-PDAP-ICG-Met@PM)通过Fe-PDAP核和封装的ICG促进了磁共振成像(MRI)和荧光成像。本研究提出了一种利用仿生纳米系统改进PDT和靶向癌症治疗的方法,为有效的肿瘤抑制提供了创新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Platelet Membrane-Coated Drug-Loaded Nanoparticles for Dual-Modal Imaging and Photodynamic Therapy in Triple-Negative Breast Cancer
Photodynamic therapy (PDT) has emerged as a promising strategy for cancer treatment; however, its efficacy is hindered by the hypoxic tumor microenvironment. In this study, we designed a bionic nanosystem to enhance oxygen-dependent PDT. The system comprises Fe-doped polydiaminopyridine (Fe-PDAP) nanoenzymes with catalase-like activity, encapsulated with metformin (Met) and indocyanine green (ICG). The surface of the nanoparticles was further coated with platelet membranes (PM), enabling targeted delivery to the tumor site via molecular recognition. In vitro and in vivo studies demonstrated that Fe-PDAP nanoenzymes catalyzed the generation of O2 from elevated hydrogen peroxide (H2O2) while concurrently depleting glutathione (GSH), resulting in increased production of reactive oxygen species (ROS) and enhanced PDT efficacy. Met, acting as a mitochondrial respiratory inhibitor, disrupts complex I of the electron transport chain, thereby reducing ATP levels, inhibiting oxygen (O2) consumption at the tumor site, and amplifying the PDT effect. Additionally, the bionic nanoparticles (Fe-PDAP-ICG-Met@PM) facilitated both magnetic resonance imaging (MRI) and fluorescence imaging via the Fe-PDAP core and the encapsulated ICG. This study presents an approach to improve PDT and targeted cancer therapy by using bionic nanosystems, providing innovative strategies for effective tumor inhibition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


