铜阴极上共轭芳醛电催化碳-碳偶联机理的研究
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在这项工作中,我们证明了Cu有效地催化了共轭芳香醛(如苯甲醛)的电还原C-C偶联。我们证明,在脂肪族醛(如戊醛)、非共轭芳香醛(如氢肉桂醛)或共轭非芳香醛(如巴豆醛)中没有观察到C-C偶联。事实上,只有共轭芳醛在Cu上发生C-C偶联,这表明了它们的平面结构的重要性,因此,它们与金属表面的强相互作用。这种直接的相互作用使电子转移最终导致与反应中物理吸附的醛分子的亲电碳发生eely - ideal (ER)型反应。另外两个因素对于C-C偶联途径是不可缺少的:(i)羰基氧优先的第一次氢化导致羟基中间体的形成(即ArCHOH*),以及(ii)相对缓慢的第二次氢加成导致表面上稳定的羟基中间体。相反,当涉及其他金属或非共轭醛时,羰基碳的优先氢化,快速的第二H加成或C-C键形成的高内在屏障抑制了C-C偶联途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
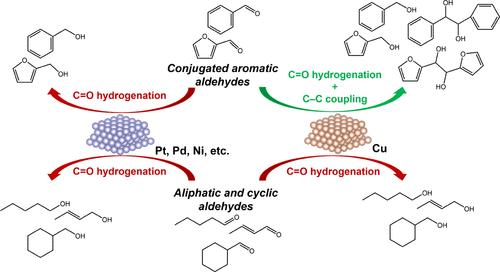
On the Mechanism of Electrocatalytic Carbon–Carbon Coupling of Conjugated Aromatic Aldehydes on Cu Cathodes
In this work, we show that Cu effectively catalyzes the electroreductive C–C coupling of only conjugated aromatic aldehydes (like benzaldehyde). We demonstrate that C–C coupling is not observed for aliphatic aldehydes (like pentanal), nonconjugated aromatic aldehydes (like hydrocinnamaldehyde), or conjugated nonaromatic aldehydes (like crotonaldehyde). The fact that only conjugated aromatic aldehydes undergo C–C coupling on Cu points to the importance of their planar structure and, consequently, their strong interaction with the metal surface. This direct interaction enables electron transfer that eventually leads to the Eley–Rideal (ER)-type reaction with the electrophilic carbon of the reacting physisorbed aldehyde molecule. Two additional factors are indispensable for the C–C coupling pathway: (i) the preferential first hydrogenation of the carbonyl oxygen resulting in the formation of a hydroxy intermediate (i.e., ArCHOH*), and (ii) a relatively slow second H addition resulting in a stable hydroxy intermediate on the surface. In contrast, when other metals or nonconjugated aldehydes are involved, preferential hydrogenation of the carbonyl carbon, fast second H addition, or a high intrinsic barrier for C–C bond formation inhibits the C–C coupling pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


