级联加氢和氢甲酰化反应的有序单活性位点
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
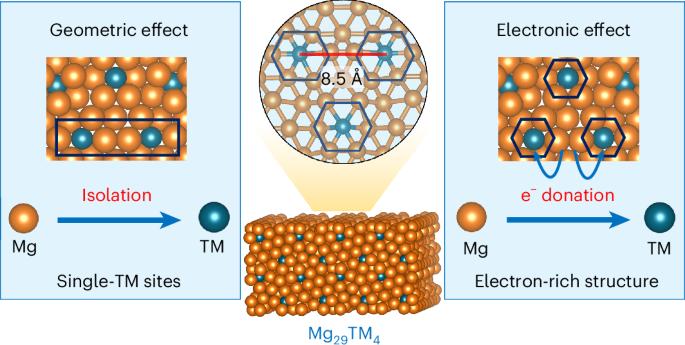
与大块金属相比,金属单原子催化剂由于其独特的电子和配位性质而具有更高的活性和选择性。然而,由于与电负性元素或活性较低的金属成键,许多单原子催化剂的活性位点随机分散,给电子能力有限。在这里,我们证明了富镁金属间化合物Mg29TM4 (TM = Pd, Rh, Ir, Pt)纳米催化剂克服了这些限制。这些材料的特点是在均匀的化学环境中周期性地分散、富含电子的贵金属单原子位。Mg29TM4在C2H2半氢化反应(Mg29Pd4)和烯烃氢甲酰化反应(Mg29Rh4)中表现出较高的活性和选择性,其中Mg29Rh4对支链醛(支链:线性>; 200:1)具有较高的区域选择性。动力学和密度泛函理论研究表明,Mg-TM系综可以精确控制碳-碳多键吸附和活化,提高活性和选择性。此外,三元Mg29Pd1.3Rh2.7催化剂具有协同作用的Mg-Pd和Mg-Rh双单原子位点,可有效催化苯乙炔加氢和氢甲酰化的级联反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Ordered single active sites for cascade hydrogenation and hydroformylation reactions
Metal single-atom catalysts offer improved activity and selectivity due to their unique electronic and coordination properties compared with bulk metals. However, many single-atom catalysts suffer from randomly dispersed active sites and limited electron-donating ability due to bonding with electronegative elements or less reactive metals. Here we demonstrate that Mg-rich intermetallic Mg29TM4 (TM = Pd, Rh, Ir, Pt) nanocatalysts overcome these limitations. These materials feature periodically dispersed, electron-rich single-atom sites of noble metals within a uniform chemical environment. Mg29TM4 exhibits high activity and selectivity in C2H2 semihydrogenation (Mg29Pd4) and olefin hydroformylation (Mg29Rh4), with Mg29Rh4 achieving high regioselectivity for branched aldehydes (branched:linear > 200:1). Kinetic and density functional theory studies suggest that the Mg–TM ensemble enables precise control over carbon–carbon multiple bond adsorption and activation, enhancing both activity and selectivity. Furthermore, the ternary Mg29Pd1.3Rh2.7 catalyst, with its synergistic Mg–Pd and Mg–Rh dual single-atom sites, efficiently catalyses a cascade reaction involving phenylacetylene hydrogenation followed by hydroformylation. Single-atom catalysts commonly present a random distribution of the active metal centres. Now a series of Mg-rich intermetallic compounds is introduced to enable ordered dispersed active metals. Furthermore, a cascade process is demonstrated on Mg29Pd1.3Rh2.7, where Pd sites catalyse the semihydrogenation of phenylacetylene with subsequent hydroformylation on Rh sites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


