肿瘤抗原优先来源于黑色素瘤和非小细胞肺癌中未突变的基因组序列。
IF 28.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
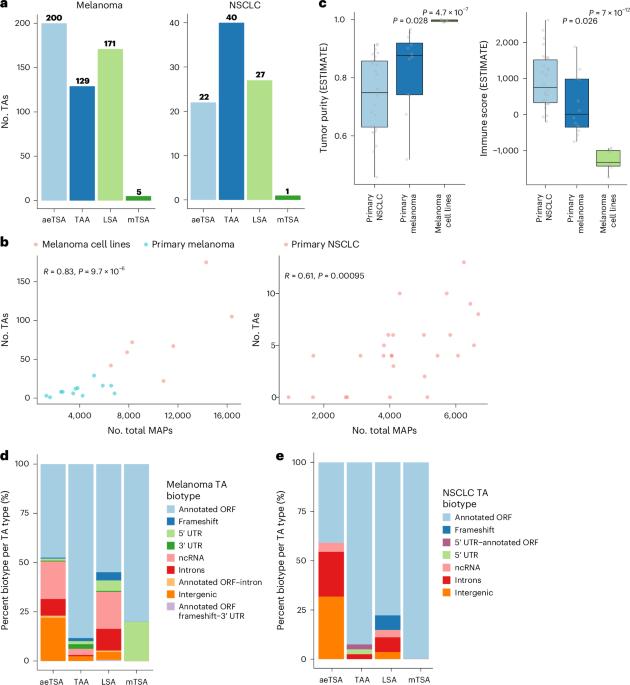
黑色素瘤和非小细胞肺癌(NSCLC)表现出异常高的突变负担。因此,这些癌症的免疫靶向主要集中在预测来自非同义突变的肿瘤抗原(TAs)上。通过全面的蛋白质基因组分析,我们在皮肤黑色素瘤(n = 505)和非小细胞肺癌(n = 90)中发现了589个TAs。其中,只有1%来自突变序列,这可以解释为大多数非同义突变的低RNA表达以及它们定位在主要组织相容性复合体(MHC) i类相关肽生成精通的基因组区域之外。相比之下,99%的TAs起源于癌症特异性的未突变基因组序列(异常表达的肿瘤特异性抗原(aeTSAs), n = 220),在癌症中过度表达(肿瘤相关抗原(TAAs), n = 165)或特异性的细胞起源谱系(谱系特异性抗原(LSAs), n = 198)。aetsa的表达受表观遗传调控,大部分由非规范基因组序列编码。aetsa在肿瘤样本中是共享的,具有免疫原性,可以促进对先前研究中观察到的免疫检查点封锁的反应,支持它们在癌症中的免疫靶向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tumor antigens preferentially derive from unmutated genomic sequences in melanoma and non-small cell lung cancer
Melanoma and non-small cell lung cancer (NSCLC) display exceptionally high mutational burdens. Hence, immune targeting in these cancers has primarily focused on tumor antigens (TAs) predicted to derive from nonsynonymous mutations. Using comprehensive proteogenomic analyses, we identified 589 TAs in cutaneous melanoma (n = 505) and NSCLC (n = 90). Of these, only 1% were derived from mutated sequences, which was explained by a low RNA expression of most nonsynonymous mutations and their localization outside genomic regions proficient for major histocompatibility complex (MHC) class I-associated peptide generation. By contrast, 99% of TAs originated from unmutated genomic sequences specific to cancer (aberrantly expressed tumor-specific antigens (aeTSAs), n = 220), overexpressed in cancer (tumor-associated antigens (TAAs), n = 165) or specific to the cell lineage of origin (lineage-specific antigens (LSAs), n = 198). Expression of aeTSAs was epigenetically regulated, and most were encoded by noncanonical genomic sequences. aeTSAs were shared among tumor samples, were immunogenic and could contribute to the response to immune checkpoint blockade observed in previous studies, supporting their immune targeting across cancers. Based on a proteogenomic analysis, Perreault and colleagues report that the majority of predicted tumor antigens originate from unmutated genomic sequences in melanoma and non-small cell lung cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


