以Isatins为原料合成1,4-苯二氮卓-2-酮
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
各种受n保护的氨基酸与isatin偶联形成n -氨基酰化蛋白。这些新型n -酰化蛋白与醇或胺发生开环反应,生成相应的n -乙氧基酯或酰胺。脱保护后,在基本条件下环化产生1,4-苯二氮卓-2-酮,在C5位置具有酯或酰胺官能团。该方法为构建具有不同C3和C5取代基的苯二氮卓类衍生物提供了有效途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
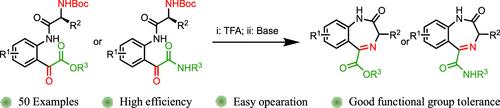
Synthesis of 1,4-Benzodiazepin-2-ones from Isatins
Various N-protected amino acids were coupled to isatin forming N-amino acylisatins. These novel N-acylisatins participate in a ring–opening reaction with either alcohols or amines to give the corresponding N-glyoxylesters or amides. Upon deprotection, cyclization under basic conditions produces 1,4-benzodiazepin-2-ones bearing ester or amide functionalities at the C5 position. This method provides an effective approach for the construction of benzodiazepine derivatives with diverse substituents at C3 and C5.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


