铑催化不对称Si-H / O-H脱氢偶联的机理:揭示si -立体硅烷合成中反应性和对映选择性的起源
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
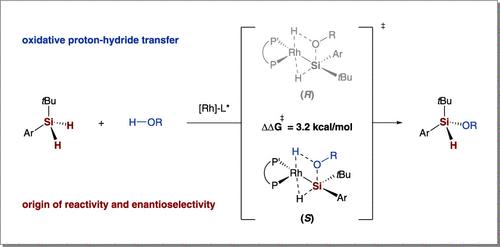
通过动力学研究和DFT计算,我们阐明了铑催化的二氢硅烷与醇的不对称脱氢偶联反应性和对映选择性的机理来源。sn2型氧化质子-氢化物转移(SN2-OPHT)途径是主要的反应机制,与传统的氧化加成和σ键转化途径相比,具有较低的能垒。关键是,二氢硅烷中的第二个Si-H键发挥了独特的作用,在单氢硅烷控制实验中完全抑制了反应性。畸变/相互作用分析进一步表明,高对映体选择性源于竞争过渡态之间的不同空间畸变。这些发现不仅合理化了硅立体硅烷合成的立体化学结果,而且为通过精确的过渡态调制设计对映选择性转化建立了框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanistic Insights into Rh-Catalyzed Asymmetric Si–H/O–H Dehydrocoupling: Unraveling the Origins of Reactivity and Enantioselectivity in Si-Stereogenic Silanes Synthesis
Through combined kinetic studies and DFT calculations, we elucidate the mechanistic origins of the reactivity and enantioselectivity in rhodium-catalyzed asymmetric dehydrogenative coupling between dihydrosilanes and alcohols. An SN2-type oxidative proton-hydride transfer (SN2-OPHT) pathway was identified as the dominant mechanism, exhibiting a lower energy barrier compared to conventional oxidative addition and σ-bond metathesis pathways. Crucially, the second Si–H bond in dihydrosilanes was revealed to play a unique role, as evidenced by the complete suppression of the reactivity in monohydrosilane control experiments. Distortion/interaction analysis further demonstrated that the high enantioselectivity arises from differential steric distortions between competing transition states. These findings not only rationalize the stereochemical outcomes in Si-stereogenic silane synthesis but also establish a framework for designing enantioselective transformations through precise transition state modulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


