开环复分解聚合中末端烯烃空前的反应活性
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在金属催化的烯烃转化反应中,脂肪族末端烯烃的使用是普遍存在的。因此,多年来人们对这类烯烃的反应性进行了大量的机理和动力学研究。但当涉及开环复分解聚合(ROMP)化学时,这种烯烃作为有效的区域选择性链转移剂(CTA)的使用从未实现过。在这里,我们研究了使用常用的降冰片烯-亚胺基单体的传统ROMP中末端烯烃的反应性出乎意料的高,尽管非常受控。1H NMR谱和MALDI-ToF质谱分析表明,该烯烃在ROMP中具有良好的区域选择性和化学选择性。研究了烯烃cta的电子参数和空间参数。动力学测量结果显示,迄今为止所报道的cta中链转移速率常数最高。此外,观察到末端烯烃浓度对聚合速率的不寻常的独立性,这归因于更高的链转移和再引发步骤的速率。这种方法描述了廉价、功能性和丰富的末端烯烃是ROMP工艺中令人惊讶的更好的cta,该工艺使用亚化学计量量的金属催化剂来形成聚合物链的数量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
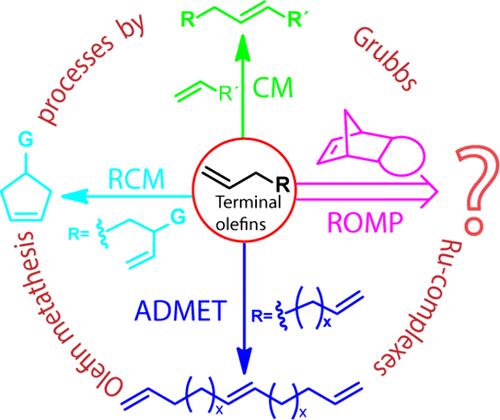
Unprecedented Reactivity of Terminal Olefins in Ring-Opening Metathesis Polymerization
The use of aliphatic terminal olefins is ubiquitous in metal-catalyzed olefin metathesis reactions. Therefore, a great deal of mechanistic and kinetic studies have been performed regarding the reactivity of such olefins over the years. But when it comes to ring-opening metathesis polymerization (ROMP) chemistry, the use of such olefins as an effective and regioselective chain transfer agent (CTA) has never been realized. Here, we investigated the unexpectedly high, albeit very controlled, reactivity of terminal olefins in traditional ROMP employing popular norbornene-imide-based monomers. 1H NMR spectroscopy and MALDI-ToF mass spectrometry analyses strongly suggested the desired regioselectivity as well as the chemoselectivity of such olefins in ROMP. Both electronic and steric parameters of the olefin CTAs are also investigated. Kinetic measurements revealed the highest chain transfer rate constants among the reported CTAs to this date. Moreover, an unusual independence of the terminal olefin concentration on the polymerization rate was observed, which was attributed to the higher rates of both chain transfer and reinitiation steps. This approach describes inexpensive, functional, and abundant terminal olefins as surprisingly better CTAs for a ROMP process that utilizes substoichiometric amounts of metal catalyst with respect to the number of polymer chains formed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


