肠神经系统在艰难梭菌感染发病机制中的作用
IF 51
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
艰难梭菌是全世界抗生素相关性腹泻的主要病因。在美国,艰难梭菌感染(CDI)是所有胃肠道疾病中第八大再入院原因和第七大死亡原因。胃肠道运动障碍和/或腹泻发生在CDI急性期后,但感染后持续的胃肠道功能障碍支持肠神经系统(ENS)神经可塑性的贡献,在CDI自然过程中,ENS在调节肠道运动和分泌中起关键作用。在这里,我们的目标是提供最新的总结肠内皮系统和肠道外源性神经支配如何受到CDI的影响,以及肠内皮系统反应如何促进CDI的发病机制和结果。在人类和临床前模型中,肠神经元和胶质细胞是艰难梭菌毒素的靶点,内皮系统和外源性神经支配的改变会导致肠道炎症、损伤和分泌性腹泻。这些发现提示CDI与ENS之间可能存在双向相互作用,更多的研究将集中于了解ENS和外源神经元释放的各种神经递质和介质如何调节对CDI的免疫反应,从而为平衡宿主反应、改善CDI管理和预防感染后胃肠道功能障碍提供新的药理学方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
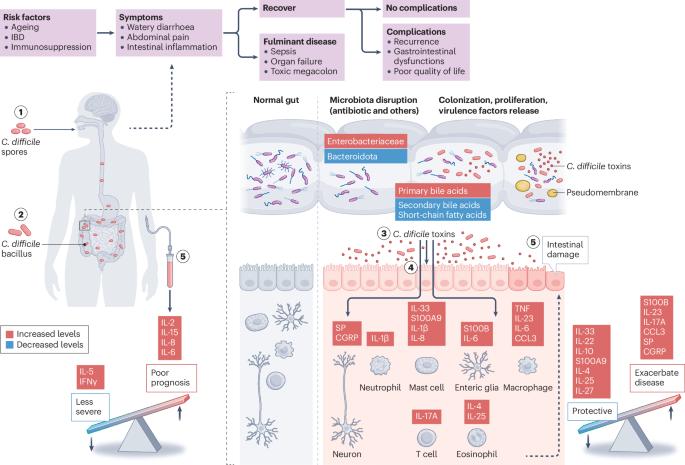

The role of the enteric nervous system in the pathogenesis of Clostridioides difficile infection
Clostridioides difficile is the leading cause of antibiotic-associated diarrhoea worldwide. In the USA, C. difficile infection (CDI) is the eighth leading cause for hospital readmission and seventh for mortality among all gastrointestinal disorders. Gastrointestinal dysmotility and/or diarrhoea occurs after the acute phase of CDI, but persistent gastrointestinal dysfunction post-infection supports contributions of neuroplasticity in the enteric nervous system (ENS), which has a key role in regulating intestinal motility and secretion, in the natural course of CDI. Here, our goal is to provide an up-to-date summary of how the ENS and extrinsic innervation of the gut are affected by CDI and how ENS responses contribute to CDI pathogenesis and outcomes. Enteric neurons and glia are targets of C. difficile toxins in humans and in preclinical model, and changes to the ENS and extrinsic innervation contribute to intestinal inflammation, damage and secretory diarrhoea. These findings suggest possible bidirectional interaction between CDI and the ENS. More studies focusing on understanding how various neurotransmitters and mediators released by the ENS and extrinsic neurons modulate immune responses to CDI could provide insight into novel pharmacological approaches to balance the host response, improve the management of CDI and prevent gastrointestinal dysfunction post-infection. This Perspective highlights emerging evidence of an interaction between Clostridiodes difficile and the enteric nervous system (ENS) during infection, discussing underlying mechanisms and how the ENS and extrinsic innervation are affected by C. difficile infection and toxins and how ENS responses contribute to pathogenesis and disease outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
52.30
自引率
0.60%
发文量
147
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Gastroenterology & Hepatology aims to serve as the leading resource for Reviews and commentaries within the scientific and medical communities it caters to. The journal strives to maintain authority, accessibility, and clarity in its published articles, which are complemented by easily understandable figures, tables, and other display items. Dedicated to providing exceptional service to authors, referees, and readers, the editorial team works diligently to maximize the usefulness and impact of each publication.
The journal encompasses a wide range of content types, including Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements, all pertinent to gastroenterologists and hepatologists. With its broad scope, Nature Reviews Gastroenterology & Hepatology ensures that its articles reach a diverse audience, aiming for the widest possible dissemination of valuable information.
Nature Reviews Gastroenterology & Hepatology is part of the Nature Reviews portfolio of journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


